Alemtuzumab is used in the treatment of multiple sclerosis, which was primarily invented as an anticancer agent, but is now widely used in MS. It is used in the form of injections, where the course lasts about 12 weeks. Treatment is stopped if symptoms begin to progress further. It also has a number of side effects, which are also important to familiarize yourself with.
MS therapy is a very long process. It is very important that the patient is prepared for the fact that drug therapy can continue even throughout life.
Only today there is a wide range of pharmaceuticals that can stop the various manifestations that the patient suffers from. MS has a chronic course that affects many parts of the central nervous system. The disease has a negative effect on myelin, which in turn protects the nerves from pathological effects on them.
The disease cannot be cured, however, it can be significantly weakened, for which the most advanced and effective medications are used, for example, for multiple sclerosis, mildronate, immunoglobulins, beta blockers and other tablets for multiple sclerosis are used, which we will discuss in more detail. In this article we will try to talk about the most common medications in the treatment of this pathology.
How does Lemtrada work?
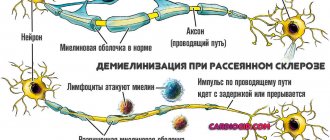
Multiple sclerosis is an autoimmune disease in which the immune system attacks the sheaths of nerve fibers necessary for the normal conduction of impulses. The disease is chronic and is currently considered incurable; doctors can only slow down its progression.
The immune system is complex. It consists of different types of cells, they “communicate” with each other and with other tissues of the body using molecular signals. There are special receptor proteins on the surface of immune cells. When they receive a signal and are activated, it leads to certain effects.
One of these receptors is called CD52 (another name is CAMPATH-1 antigen). Leukocytes that carry it on their surface are prone to aggression against the nervous system. They are credited with a leading role in the occurrence of relapsing multiple sclerosis.
Lemtrada attaches to white blood cells that carry the CD52 receptor and causes “genocide” of them. The body is cleansed of “pest leukocytes”, and gradually their place is taken by “useful” immune cells.
This mechanism of action has been studied in transgenic mice. The subtleties of how the drug works in the human body are still not fully known.
Who is treated with Lemtrada and how?
The most effective therapy for multiple sclerosis is currently considered to be regimens using DMTs - drugs that change the course of multiple sclerosis. They usually need to be administered every few days or monthly.
When treating with Lemtrada, only two short courses over two years are sufficient:
- The first course lasts 5 days in a row. Every day the drug is administered intravenously over 4 hours, after which the patient’s condition is monitored for 2 hours.
- The second course is carried out a year later, it lasts 3 days.
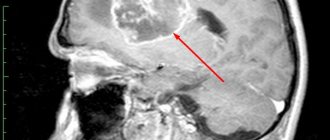
The main indication for the use of Lemtrada, as we have already mentioned, is relapsing multiple sclerosis, if, despite treatment with other drugs, the patient has had a relapse within the last year or has deteriorated according to MRI results.
Alemtuzumab is sometimes used as a first-line treatment if a person with multiple sclerosis has recently relapsed or an MRI has detected active disease (new lesions in the brain).
Innovative drug Alemtuzumab for the treatment of multiple sclerosis
Why is this new medicine needed? An experienced neurologist will be able to answer this question in detail for each patient. Representing a group of antitumor solutions, Alemtuzumab was created on the basis of a monoclonal antibody.
It was developed in the USA (Cambridge University) back in the late 70s of the last century. The drug is prescribed by doctors to effectively influence white blood cells that are affected by cancer and spoil the blood. The medicine is recommended for chronic manifestations of multiple sclerosis and is prescribed in a neurological clinic.
Refers to experimental designs containing monoclonal antibodies. It is used in the treatment of multiple sclerosis, characterized by a relapsing-remitting condition of the patient.
This type of MS (multiple sclerosis) is characterized by wave-like periods of exacerbation. Therefore, when choosing the most effective drugs to reduce inflammation in the central nervous system, one should not forget about innovative developments from this group of drugs.
Consult your doctor for dosages!
The drug is new and difficult to use, which means the dosage and treatment regimen should be obtained from the attending physician, who will be able to monitor the patient’s condition and correctly prescribe a course of treatment.
It is important to have significant experience in conducting antitumor therapy, then the dosage and method of use of this ingredient will not be difficult to determine.
Much depends on the form of release, as well as the diagnosis of concomitant diseases, the degree of neglect of the identified relapse.
When treating with this medicine, the clinic of elderly patients is carefully monitored.
Overdose and additional instructions
Considering the side effects of the drug, experts state that patients who received a repeat dose up to a volume of 240 mg experienced an overdose. In such situations, record:
- arterial hypotension;
- signs of fever;
- significant anemia (grade III–IV, count according to NCI).
Signs of an overdose may be characterized by fever and allergic reactions of the body. In such situations, the drug is discontinued; no specific antidote has been identified.
Possible analogues (substitutes)
Campas Show all analogues of the drug Lemtrada » Attention: the use of analogues must be agreed with the attending physician.
Active substance, group
Alemtuzumab, monoclonal MIBP antibodies
Dosage form
Concentrate for the preparation of solution for infusion
How to use: dosage and course of treatment
IV infusion, for at least 2 hours.
In the first week of treatment, increasing doses are prescribed - 3 mg on the 1st day, 10 mg on the 2nd day and 30 mg on the 3rd day, provided that each dose is well tolerated. Next - 30 mg per day 3 times a week (every other day). The maximum duration of treatment is 12 weeks.
In most patients, increasing the dose of the drug to 30 mg can be done within 3-7 days.
However, if severe adverse reactions develop (especially decreased blood pressure, chills, fever or bronchospasm) at both a dose of 3 mg and 10 mg, the next daily dose of the drug should be the same and should not be increased until the drug is well tolerated .
Source: https://yazdorov.win/nevralgiya/innovatsionnyj-preparat-alemtuzumab-dlya-lecheniya-rasseyannogo-skleroza.html
How much better is this than classical therapy?
The results of one of the largest studies were published in the authoritative scientific journal The Lancet. The authors concluded that when treated with Lemtrada, the risk of relapse was 54.9% lower than when using a drug from the DMT group - interferon beta-1a (Rebif). It was the publication in The Lancet that largely contributed to the fact that in November 2014 the drug was approved in the United States for the treatment of multiple sclerosis.
Similar results were noted in other studies.
With regard to the risk of disability, the situation turned out to be ambiguous. In some studies, alemtuzumab was 42% better than interferon. In others, the difference was 30% - this figure could be due not to drugs, but to random reasons.
Alemtuzumab. Clinical researches
Alemtuzumab is another drug currently in clinical trials for the treatment of relapsing-remitting MS.
The UK has published a fact sheet for people with multiple sclerosis, which provides basic information about alemtuzumab (Campas).
The purpose of this document is to inform the patient as much as possible about the drug for subsequent discussion with the attending physician. Here are key excerpts from the newsletter:
What is alemtuzumab?
Alemtuzumab (Campas) is an experimental drug that is currently undergoing clinical trials to study its use in the treatment of patients with relapsing-remitting MS. Although alemtuzumab is not currently registered for the treatment of MS, it is registered for the treatment of chronic lymphocytic leukemia (cancer).
How does alemtuzumab work?
Alemtuzumab is a humanized monoclonal antibody drug. Antibodies are proteins produced by the immune system to fight foreign bodies such as infections. Monoclonal antibodies are produced in the laboratory using genetic engineering. Once in the human body, they specifically bind to proteins, thereby changing the immune response.
Alemtuzumab works by neutralizing T cells, which are part of the immune system. In multiple sclerosis, T cells mistakenly attack myelin and cause inflammation, which can be seen on MRI. It is believed that the T cells that recover after treatment with alemtuzumab do not include the subset that destroys myelin.
MS and clinical trials of alemtuzumab
The first clinical studies of Alemtuzumab were undertaken for the treatment of two types of MS: relapsing-remitting and secondary progressive multiple sclerosis.
In patients with relapsing-remitting MS, Alemtuzumab showed a significant reduction in the number of relapses and no increase in disability. No increase in disability was observed for three years after treatment.
However, more questionable results were observed in 25 people diagnosed with secondary progressive multiple sclerosis. Although MRI in these patients for seven years did not show the formation of new lesions in the brain and spinal cord, disability continued to accumulate in all of them.
This has led researchers to hypothesize that something other than inflammation influences progression in secondary progressive MS. Subsequent studies were aimed at studying the effect of alemtuzumab in the early stages of relapsing-remitting MS.
Results from a phase II clinical trial of Alemtuzumab (known as CAMMS-223) were published in October 2008. The study included 334 patients with relapsing-remitting MS. Participants were randomized into three groups to receive either high-dose Alemtuzumab, low-dose Alemtuzumab, or beta-interferon 1a (Rebif).
Alemtuzumab was administered by intravenous infusion in annual courses: in the first year of treatment, it was administered once daily for five consecutive days, then 12 months later, it was administered once daily for three days.
A third course of three infusions was scheduled after another 12 months, however not all participants received this course due to safety concerns (see Side Effects and Contraindications). Interferon beta 1a was administered subcutaneously three times a week.
All study participants received intravenous corticosteroids (methylprednisolone) for three days every 12 months.
The results showed that patients treated with Alemtuzumab experienced significantly fewer relapses than patients treated with interferon-1a beta during the study period.
Over three years, 77% of patients treated with low-dose Alemtuzumab and 84% of patients treated with high-dose Alemtuzumab experienced no relapses, compared with 52% of patients treated with interferon beta-1a.
The study results also showed that, compared with interferon beta-1a, Alemtuzumab reduced the risk of permanent disability by 71%.
Results from a five-year follow-up of patients in this study were presented at the annual meeting of the American Academy of Neurology in April 2011.
They showed that nearly two-thirds of patients who received Alemtuzumab were free of clinical disease activity (defined as both absence of relapses and no sustained increase in disability as measured by the Expanded Disability Scale) for four years after the last course of treatment, compared with 27 % of patients receiving interferon beta-1a. However, patients who received any of these drugs and whose disease worsened during the early years of the study were excluded from subsequent studies.
Side effects and contraindications
Low blood platelet counts were observed in 3% of study participants. Although this side effect is potentially serious, the condition is treatable in its early stages.
However, the use of Alemtuzumab in a phase II clinical trial was temporarily suspended in September 2004 after the death of one participant due to idiopathic thrombocytopenic purpura (ITP).
ITP is a disease caused by a low number of platelets in the blood and a resulting bleeding disorder. Its symptoms include purple bruises on the skin and mouth. ITP can cause internal bleeding.
Following the disaster, new strategies were put in place to ensure that all cases of ITP were identified early. However, the risk of developing ITP remains significant.
22.6% of study participants experienced side effects related to thyroid dysfunction. These disorders are treatable, but it is possible that as a result of these disorders, thyroid treatment may be lifelong.
Also, after the infusion, an increase in the number of colds and infectious diseases was recorded. Due to the fact that the action of Alemtuzumab is associated with suppression of the immune system, a person becomes more vulnerable to infections such as flu and colds.
The published results of the second phase of the study discussed issues related to the treatment of patients in the early stages of multiple sclerosis:
Although our study shows that Alemtuzumab is a more effective drug than interferon beta-1a when administered in the earliest stages of relapsing-remitting multiple sclerosis, our findings raise difficult questions about the appropriateness of this drug in younger patients who have minimal disability due to potentially serious side effects. Our study did not evaluate long-term safety, but rather was designed to identify unusual adverse reactions.
Current clinical trials
The phase III clinical trial of Alemtuzumab, known as CARE-MS1 or CAMMS-323 (Comparing the Efficacy of Alemtuzumab and Rebif), is to compare the effectiveness of low-dose alemtuzumab with interferon-beta-1a over two years. This phase II continuation study began in the UK and US in 2007. This study is expected to be completed in mid-2011.
The second study, CARE-MS2 or CAMMS-324, involving 200 people who had no significant response to disease-modifying drugs (Avonex, Betaferon, Copaxone or Rebif), compared the effectiveness of two dosing options for Alemtuzumab . This study will also compare the effects of Campas and Rebif on cognitive symptoms in MS (eg, attention, memory, speed of thinking). This study is expected to be completed by the end of 2011.
Studying and evaluating the long-term safety and effectiveness of Alemtuzumab is also part of the CAMMS-223, CAMMS-323 and CAMMS-324 studies. Results from these three studies are expected in late 2011 to mid-2012.
Source: https://ProSkleroz.ru/klinicheskie-issledovaniya-2/alemtuzumab-klinicheskie-issledovaniya
Risks associated with drug administration
Lemtrada is not a safe drug. Treatment is associated with certain risks. Some side effects may be life-threatening:
- Autoimmune conditions in which the immune system begins to attack the body's own tissues. With immune thrombocytopenia, increased bleeding develops, with kidney damage - severe renal failure, which may require dialysis or a kidney transplant.
- Reactions to drug administration develop within 24 hours (sometimes later) after infusion and can be very severe. Alarming symptoms that require immediate medical attention: swelling of the face, difficulty breathing, severe weakness, increased or slow heartbeat, a feeling as if the heart is “freezing”, “turning over”, “jumping out of the chest”, chest pain, rash on skin.
- Oncological diseases. Treatment with Lemtrada increases the risk of thyroid cancer, melanoma, and lymphoma.
- Problems with the thyroid gland. It is possible to both increase (hyperthyroidism) and decrease (hypothyroidism) its function. “Alarm bells”: severe sweating, unreasonable weight loss or gain, rapid heartbeat, nervousness, emotional instability, increased fatigue, chilliness in the arms and legs, constipation.
- Blood problems. Due to a decrease in the number of different types of cells in the blood, some disorders develop: frequent infections, jaundice, chest pain, rapid heartbeat, dark urine.
- Severe infections. The likelihood of infection with herpes, hepatitis, and tuberculosis viruses increases.
- Lung disorders. Some patients receiving alemtuzumab develop pneumonitis, a non-infectious inflammation of the lungs.
What is plasmapheresis?
Plasmapheresis is used to purify the patient's blood. The essence of this procedure is that the blood is passed through a special apparatus, where the blood plasma is purified at the cellular level. Thus, the plasmapheresis procedure allows you to cleanse the blood of many pathological and toxic substances, which has a beneficial effect on tissues, organs and the functioning of systems throughout the body. That is why patients after a course of treatment note a surge of vigor, strength and energy. Symptoms of chronic diseases that have tormented patients for years disappear. Metabolic processes are activated, excess weight disappears, swelling, constipation and other stagnation disappear, complexion improves, skin tightens, sleep normalizes, etc.
This method of blood purification is very often carried out in case of acute intoxication - food and alcohol poisoning, also burn disease, infectious diseases of the joints and bones. It is also used for diseases such as diabetes, sepsis, bronchial asthma, infertility, chronic hepatitis, liver failure, as well as atherosclerosis and coronary heart disease.
What is important to tell your doctor before starting treatment?
The risk of serious side effects can be reduced by taking the drug exactly as directed and not using it on people who already have health problems. If the patient suffers from at least one of the following conditions, the doctor must know about it:
- increased bleeding;
- dysfunction of the thyroid gland;
- renal dysfunction;
- HIV;
- any infections, including recent ones;
- cancer;
- vaccination with live vaccine within the previous 6 weeks.
Pregnancy and breastfeeding are also contraindications to the use of the drug. If you take any medications, vitamins, dietary supplements, be sure to tell your doctor about it.
We offer comprehensive rehabilitation for multiple sclerosis. For detailed information call us at number. We will arrange a consultation with recognized experts in the treatment of multiple sclerosis as soon as possible.
What drugs are used as DMTs?
Modern PITS must meet certain requirements. Firstly, they must not only be quite effective, but also safe, because they will take a long time to be treated.
Secondly, it is important that the patient strictly follows the doctor’s recommendations, and for this, taking the drug should be simple and convenient.
It is better if the medicine can be taken in tablet form. If it is prescribed in the form of injections, they should not be frequent.
Drugs that usually begin treatment of multiple sclerosis (first-line drugs):
- Interferon beta-1a (Rebif) for subcutaneous administration (three times a week).
- Interferon beta-1a (Avonex) for intramuscular administration (weekly).
- Interferon beta-1b (betaferon) for subcutaneous administration (every other day).
- Glatiramer acetate (Copaxone) for subcutaneous administration (daily).
- Teriflunamide (Abagio) tablet drug (daily).
These drugs are the most well studied. They help many patients, but sometimes they are not effective enough. In such cases, the doctor may consider using second-line DMTs:
- Natalizumab (Tysabri) - intravenous drip (once every 28 days).
- Mitoxantrone (Novatron Oncotron) - intravenous drip (once every three months).
- Fingolimod (Gilenia) is a tablet drug (daily).
Second-line drugs have a number of serious side effects - cardiotoxicity, heart rhythm disturbances, and the appearance of PML (progressive multifocal leukodystrophy).
Therefore, a thorough analysis is required - the so-called risk stratification, as well as regular monitoring of patients when prescribing these drugs.
concentrate for the preparation of solution for infusion