Has restrictions during pregnancy
Has restrictions when breastfeeding
Allowed for children
Has restrictions for older people
Prohibited for liver problems
Has limitations for kidney problems
Depakine Chronosphere belongs to the group of drugs that suppress epileptic seizures. In addition to its antiepileptic effect, it produces a calming effect and leads to relaxation of skeletal muscles. This combination of actions helps to quickly and effectively stop epileptic seizures, and when used systematically, prevent their occurrence.
Pharmacology
The main component of the drug has a sedative and central muscle relaxant effect.
The principle of operation is to increase the content of GABA (gamma-aminobutyric acid) in the central nervous system.
This process is made possible by inhibiting GABA transferase and reducing the reuptake of gamma-aminobutyric acid in the brain.
Systemic use of Depakine reduces excitability and inhibits seizures that develop in epilepsy in the motor areas of the brain.
Valproic acid stimulates GABAergic transmission and stabilizes psycho-emotional mood.
The active substance has antiarrhythmic activity. In the totality of the zones of influence, the drug can achieve a high therapeutic effect.
Indications for use
Depakine is used in pediatrics to eliminate manifestations of epilepsy of various origins.
In addition to reducing the activity of seizures, the medicine helps to correct the patient’s character and behavior, which have deviations characteristic of the disease.
The active substance provides high therapeutic results for the following problems:
- febrile convulsions, childhood tics;
- seizures occurring due to organic brain diseases;
- manic-depressive psychosis of the bipolar type;
- Lennox-Gastaut, West syndromes, etc.
Depakine shows high results not only when used for medicinal purposes, but also as a preventive measure.
When using Depakine, it is necessary to take into account such contraindications as:
- Hepatitis in any form;
- Diseases of the pancreas;
- Thrombocytopenia, leukopenia;
- Individual sensitivity of the body to the components of the drug;
- Liver dysfunction;
- Porphyria;
- Hemorrhagic diathesis;
- Pregnancy and lactation period;
- Age up to 6 months;
- Ages up to 3 years – do not prescribe the drug by injection.
Depakine should be used with caution in case of kidney disease.
Adult patients are prescribed this drug for the treatment of:
- Lennox-Gastaut syndrome;
- generalized and partial epileptic seizures;
- bipolar affective disorders.
Children are given Depakine Chrono for treatment:
- partial and generalized epileptic seizures;
- Lennox-Gastaut syndrome.
The drug should not be used for:
- hypersensitivity to the components of this drug;
- chronic hepatitis;
- hepatic porphyria;
- body weight less than 17 kg;
- acute hepatitis;
- taking Mefloquine and/or St. John's wort extract;
- children under 6 years of age.
Depakine Chronosphere, 30 pcs., 250 mg, extended-release granules
Before starting the use of Depakine® Chronosphere™ and periodically during the first 6 months of treatment, especially in patients at risk of developing liver damage, liver function tests should be performed.
As with the use of most antiepileptic drugs, when using valproic acid, a slight increase in the activity of liver enzymes is possible, especially at the beginning of treatment, which occurs without clinical manifestations and is transient. In these patients, a more detailed study of biological parameters, including the prothrombin index, may be necessary, and the dose of the drug may need to be adjusted, and, if necessary, repeated clinical and laboratory examinations.
Before starting therapy or before surgery, as well as in the event of spontaneous occurrence of subcutaneous hematomas or bleeding, it is recommended to determine the bleeding time and the number of formed elements in the peripheral blood, including platelets.
Severe liver damage
Predisposing factors.
Clinical experience shows that the risk group includes patients receiving multiple antiepileptic drugs at the same time, children under three years of age with severe seizures, especially against the background of brain damage, mental retardation and/or congenital metabolic or degenerative diseases; patients simultaneously taking salicylates (since salicylates are metabolized through the same metabolic pathway as valproic acid).
After 3 years of age, the risk of liver damage decreases significantly and decreases progressively as the patient ages. In most cases, such liver damage occurred during the first 6 months of treatment, most often between the 2nd and 12th weeks of treatment, and usually when valproic acid was used as part of combination antiepileptic therapy.
Symptoms indicating possible liver damage.
For early diagnosis of liver damage, clinical observation of patients is mandatory. In particular, you should pay attention to the appearance of the following symptoms, which may precede the onset of jaundice, especially in patients at risk (see above):
- nonspecific symptoms, especially those that began suddenly, such as asthenia, anorexia, lethargy, drowsiness, which are sometimes accompanied by repeated vomiting and abdominal pain;
- resumption of seizures in patients with epilepsy.
Patients or their family members (when using the drug in children) should be warned that they should immediately report the occurrence of any of these symptoms to their doctor. Patients should immediately undergo clinical examination and laboratory testing of liver function tests.
Identification.
Liver function tests should be performed before starting treatment and then periodically during the first 6 months of treatment. Among conventional studies, the most informative are studies reflecting the state of the protein-synthetic function of the liver, especially the determination of the prothrombin index. Confirmation of abnormal prothrombin index, especially in combination with abnormalities in other laboratory parameters (significant decrease in fibrinogen and coagulation factors, increased bilirubin concentration and increased transaminase activity), as well as the appearance of other symptoms indicating liver damage (see above ), requires discontinuation of the use of the drug Depakine® Chronosphere™. As a precaution, if patients were concomitantly receiving salicylates, their use should also be discontinued.
Pancreatitis.
There are rare reported cases of severe forms of pancreatitis in children and adults, developing regardless of age and duration of treatment. Several cases of hemorrhagic pancreatitis have been observed with rapid progression of the disease from the first symptoms to death. Young children are at increased risk of developing pancreatitis; the risk decreases as the child ages. Severe seizures, neurological disorders, or anticonvulsant therapy may be risk factors for developing pancreatitis. Liver failure combined with pancreatitis increases the risk of death. Patients who experience severe abdominal pain should be evaluated immediately. If pancreatitis is confirmed, in particular with increased activity of pancreatic enzymes in the blood, the use of valproic acid should be discontinued and appropriate treatment should be initiated.
Suicidal thoughts and attempts.
Suicidal thoughts and attempts have been reported in patients receiving antiepileptic drugs for some indications. A meta-analysis of randomized placebo-controlled trials of antiepileptic drugs also showed a 0.19% increase in the risk of suicidal thoughts and attempts in all patients taking antiepileptic drugs (including a 0.24% increase in this risk in patients taking antiepileptic drugs ), compared with their frequency in patients taking placebo. The mechanism of this effect is unknown. Therefore, patients taking Depakine® Chronosphere™ should be constantly monitored for suicidal thoughts and attempts, and if they occur, appropriate treatment should be provided. Patients and caregivers are advised to seek immediate medical attention if a patient experiences suicidal thoughts or attempts.
Carbapenems.
Concomitant use of carbapenems is not recommended (see “Interactions”).
Patients with established or suspected mitochondrial diseases.
Valproic acid can initiate or aggravate the manifestations of the patient's mitochondrial diseases caused by mutations in mitochondrial DNA, as well as in the nuclear gene encoding the mitochondrial enzyme γ-polymerase
(POLG)
.
In particular, in patients with congenital neurometabolic syndromes caused by mutations in the gene encoding polymerase γ (POLG)
, such as patients with Alpers-Huttenlocher syndrome, a higher incidence of acute liver failure and liver-related deaths was associated with the use of valproic acid .
Diseases due to γ-polymerase defects may be suspected in patients with a family history of such diseases or symptoms suggestive of their presence, including unexplained encephalopathy, refractory epilepsy (focal, myoclonic), status epilepticus, mental and physical retardation, psychomotor regression, axonal sensorimotor neuropathy, myopathy, cerebellar ataxia, ophthalmoplegia or complicated migraine with visual (occipital) aura and others. In accordance with current clinical practice, testing for mutations in the polymerase γ gene (POLG)
(see “Contraindications”).
Women of childbearing potential, pregnant women.
Depakine® Chronosphere™ should not be used in female children and adolescents, women of childbearing potential and pregnant women, unless alternative treatments are ineffective or not tolerated. This limitation is associated with a high risk of teratogenicity and mental and physical development disorders in children who were exposed to valproic acid in utero. The benefit/risk ratio should be carefully re-evaluated in the following cases: during regular review of treatment, when a girl reaches puberty and, urgently, if a woman taking valproic acid plans or becomes pregnant.
During treatment with valproic acid, women of childbearing potential should use reliable methods of contraception, and they should be informed of the risks associated with taking Depakine® Chronosphere™ during pregnancy (see "Use during pregnancy and lactation"). To help the patient understand these risks, the physician prescribing valproic acid should provide the patient with comprehensive information about the risks associated with taking Depakine® Chronosphere™ during pregnancy.
In particular, the physician prescribing valproic acid should ensure that the patient understands:
- the nature and magnitude of the risks when using valproic acid during pregnancy, in particular, the risks of teratogenic effects, as well as the risks of disorders of the mental and physical development of the child;
— the need to use effective contraception;
- the need for regular review of treatment;
- the need for urgent consultation with her doctor if she suspects that she is pregnant, or when she suspects the possibility of pregnancy.
A woman planning a pregnancy should definitely try, if possible, to switch to an alternative treatment before she attempts to conceive (see “Use during pregnancy and lactation”).
Treatment with valproic acid should be continued only after a physician experienced in the treatment of epilepsy and bipolar disorders has re-evaluated the benefits and risks of treatment.
Children. The information applies to dosage forms of the drug Depakine®, which can be taken by children under 3 years of age.
In children under 3 years of age, if the drug is necessary, it is recommended to use it in monotherapy. However, before starting treatment, you should weigh the ratio of the potential benefits of using valproic acid and the risk of liver damage and the development of pancreatitis when using it.
In children under 3 years of age, concomitant use of valproic acid and salicylates should be avoided due to the risk of hepatotoxicity.
Kidney failure.
It may be necessary to reduce the dose of valproic acid due to an increase in the concentration of its free form in the blood serum. If it is impossible to monitor plasma concentrations of valproic acid, the dose of the drug should be adjusted based on clinical observation of the patient.
Enzyme deficiency of the carbamide cycle (urea cycle).
If an enzymatic deficiency of the carbamide cycle is suspected, the use of valproic acid is contraindicated. Several cases of hyperammonemia with stupor or coma have been described in such patients. In these cases, metabolic studies should be performed before starting treatment with valproic acid (see "Contraindications").
In children with unexplained gastrointestinal symptoms (anorexia, vomiting, cases of cytolysis), a history of lethargy or coma, with mental retardation, or a family history of death of a newborn or infant, metabolic studies should be carried out before starting treatment with valproic acid, in particular the determination of ammonemia (presence of ammonia and its compounds in the blood) on an empty stomach and after meals (see “Contraindications”).
Patients with systemic lupus erythematosus.
Although it has been shown that during treatment with Depakine® Chronosphere™, dysfunction of the immune system is extremely rare, the potential benefits of its use must be compared with the potential risks when using the drug in patients with systemic lupus erythematosus.
Increase in body weight.
Patients should be warned about the risk of weight gain at the beginning of treatment, and measures, mainly dietary, should be taken to minimize this phenomenon.
Patients with diabetes.
Given the possibility of adverse effects of valproic acid on the pancreas, when using the drug in patients with diabetes mellitus, blood glucose concentrations should be carefully monitored. When testing urine for the presence of ketone bodies in patients with diabetes, it is possible to obtain false positive results, because valproic acid is excreted by the kidneys, partly in the form of ketone bodies.
Patients infected with HIV.
In vitro
, valproic acid has been shown to stimulate HIV replication under certain experimental conditions.
The clinical significance of this fact, if any, is unknown. Additionally, the significance of these in vitro
for patients receiving maximally suppressive antiretroviral therapy has not been established. However, these data should be taken into account when interpreting the results of continuous viral load monitoring in HIV-infected patients taking valproic acid.
Patients with existing carnitine palmitoyltransferase (CPT) type II deficiency.
Patients with existing CPT type II deficiency should be warned of the increased risk of rhabdomyolysis when taking valproic acid.
Ethanol.
During treatment with valproic acid, ethanol consumption is not recommended.
Other special instructions.
The inert matrix of the drug Depakine® Chronosphere™ (extended release drug) due to the nature of its excipients is not absorbed in the gastrointestinal tract; after the release of the active substances, the inert matrix is excreted in the feces.
Impact on the ability to drive vehicles and operate machinery.
Patients should be warned about the risk of developing drowsiness, especially in the case of combined anticonvulsant therapy or when combining Depakine® Chronosphere™ with benzodiazepines (see “Interactions”).
Pharmacodynamics and pharmacokinetics
The tablets are an anticonvulsant that is effective in various forms of epilepsy.
There are two known mechanisms of its action:
- valproic acid affects the GABAergic system. It increases the concentration of GABA in the central nervous system and stimulates GABAergic transmission;
- possible influence of valproate metabolites remaining in the brain. After the drug is eliminated, the level of GABA increases.
The bioavailability of the drug when administered orally is approximately 100%. The volume of distribution is primarily limited to blood and extracellular fluid. The active substance penetrates the brain and cerebrospinal fluid.
Half-life is 15-17 hours. The minimum concentration of the drug in blood serum is 40–50 mg/l. The maximum concentration is 200 mg/l; at higher levels, the dosage must be reduced.
Sustained concentration in plasma – after 3-4 days of use. Strong bonds with plasma proteins, which depend on the dosage. It is excreted mainly in the urine.
Restrictions on use
The manufacturer in the annotation indicates the following list of diseases for which Depakine is not prescribed:
- allergy to the components of the composition;
- hepatitis (in acute and chronic forms of development);
- pancreatic dysfunction;
- liver failure, porphyria;
- pathology of the hemostatic system;
- a decrease in platelet count below the standard value (with severe symptoms);
- pregnancy (first trimester);
- lactation period.
When prescribing products containing valproic acid, the age threshold is taken into account. It is forbidden to take tablets before the age of three. If therapy is necessary at an earlier age, it is recommended to use syrup.
A number of diseases have been identified for which valpoic acid-based products are used with caution.
Among the large list of pathologies: low protein content in the blood, congenital enzymopathies, liver dysfunction, inhibition of bone marrow hematopoiesis.
Nosological analogues
The list of products that differ from Depakine in composition, but are capable of achieving the same goals as the original product, is impressive. When selecting them, it is necessary to take into account not only the basic, but also additional properties of the main substance, the principle of action of the auxiliary components. These medications are administered with extreme caution as part of complex therapy.
Most often, doctors use the following drugs to combat epilepsy:
- "Carbamazepine" is a very cheap product based on the substance of the same name. Its use makes it possible to stop and prevent epileptic seizures and reduce their frequency. The product also relieves mood swings, depression, aggressiveness and irritability in people with epilepsy;
- Tebantin is an expensive but effective remedy based on gabapentin. It not only fights pathological seizure activity, but also has analgesic and neuroprotective properties, reduces anxiety levels;
- "Difenin" is a cheap but effective product, where the main substance is phenytoin. Its therapeutic result is achieved through a complex mechanism of action. It stops cramps, relieves pain and arrhythmia, and relaxes muscles. Unlike many antiepileptic drugs, the medicine does not cause drowsiness;
- Keppra is an expensive medicine from a Belgian manufacturer. Because of the price, its use is not used so often, but in most cases it works well.
Unlike other similar drugs, Difenin does not cause drowsiness.
Each product has its own characteristics, strengths and weaknesses. Years of use of the products have made it possible to establish for which forms of epilepsy which compounds are best to use.
Overdose
With an increase in the recommended dose, manifestations characteristic of intoxication are possible.
The risk of side effects also increases.

If you feel unwell or have other signs of poisoning, you should stop taking the medicine and seek medical help.
In case of acute overdose, coma with muscle hypotonia, miosis, metabolic acidosis, hyporeflexia and respiratory depression is possible.
In addition, there are known cases of increased intracranial pressure due to cerebral edema. Death is also possible.
Treatment includes gastric lavage, careful monitoring of the cardiovascular and respiratory systems, and maintaining effective diuresis. In serious situations, dialysis is performed.
Instructions for use
The dosage form is ideal for children and adults. The granules are easy to swallow, and their movement through the pharynx and esophagus does not cause discomfort. The drug can also be given to small children over 6 months of age who have already learned to consume pureed foods, as well as to adults with swallowing problems.
Instructions for use of the drug Depakine Chronosphere do not contain any special instructions regarding the time of administration. However, it is recommended to divide the daily dose into two times and consume it with meals. The drug is poured on top of food or drinks, which are first cooled to room temperature.
If the medicine is prescribed to small children, it is not recommended to add it to the bottle with a nipple, as the granules may clog the hole. After opening the package, the medicine is used immediately; long-term storage in an open form leads to a decrease in its effectiveness.
The medicine is dosed exclusively by a doctor on an individual basis. The daily dose is calculated based on the patient's age and weight. It is recommended to initially prescribe the drug at a dosage of 20 mg of sodium valproate per kilogram of body weight. The average daily dose of the drug is:
- children under 14 years of age – 30 mg/kg;
- at the age of 14 to 18 years – 25 mg/kg;
- adults weighing over 60 kg – 20 mg/kg.
Then, if necessary, the amount of drug can be rapidly increased to the minimum effective concentration.
Possible side effects and overdose
Taking the drug may cause adverse reactions. Negative consequences manifest themselves as follows:
- Hematopoietic system – anemia, thrombocytopenia, leukopenia.
- Blood coagulation system - increased bleeding due to a decrease in the number of coagulation factors.
- Nervous system – tremor, extrapyramidal disorders, stupor.
- Mentality – aggressiveness, impaired attention, hyperactivity.
- Digestive system – nausea, diarrhea, pancreatitis.
- Immune system – hypersensitivity, urticaria.
- Skin – alopecia, rash.
- Metabolic processes – hyponatremia, weight gain.
- Reproductive system – dysmenorrhea/amenorrhea.

Hyponatremia
An overdose is manifested by coma with muscle hypotonia, respiratory depression, hyporeflexia, and constriction of the pupils. The acid-base balance shifts towards lower pH and metabolic acidosis develops. In severe cases, cerebral edema may develop, and in severe poisoning, death.
Interaction with other drugs
The drug helps to enhance the effectiveness of other psychotropic drugs. Therefore, when prescribing them simultaneously, it is necessary to carefully select the dosage. When prescribing antiepileptic drugs that can activate microsomal oxidation in the liver, it must be taken into account that they can reduce the content of valproic acid in the blood plasma.
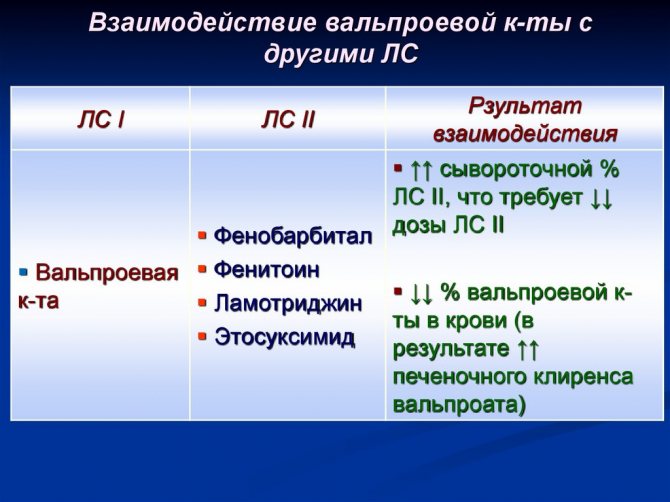
Drug interactions
Therefore, when prescribing a medication as a component of combination therapy for epilepsy, it is necessary not only to correctly calculate the dosage, but also to monitor the plasma concentration of the active substance. Simultaneous use with ethanol leads to increased hepatotoxic effects. The combined use of hormonal contraceptives and sodium valproate does not lead to a violation of the therapeutic effect of both drugs.
Side effects
During testing of the antiepileptic drug, cases of side effects were identified.
Among the common ones:
- psychosis, irritability, encephalopathy, stupor, enuresis and other central nervous system disorders;
- flickering of spots in the eyes, involuntary oscillatory movements of the eyes, disorders of the visual system;
- gastralgia, hepatitis, pancreatitis and other problems with the digestive system;
- thrombocytopenia, leukopenia, anemia and other disorders in the hematopoietic organs;
- unreasonable gain of muscle mass or its decrease;
- allergic manifestations;
- dysfunction of the endocrine system;
- alopecia, hemodialysis.
To eliminate anxiety symptoms, it is recommended to take the following measures:
- rinse the stomach;
- take sorbent;
- cause forced diuresis.
If relief is not observed, you need to call an ambulance.
Interaction
Valproic acid during complex treatment may lose its properties or cause side effects if the medications were selected incorrectly.
All appointments are made exclusively by a qualified specialist. Self-medication is fraught with serious consequences.
Depakine in combination with certain drugs reduces the therapeutic effect and neutralizes the effect of the active components of other drugs.
The annotation contains an impressive list of medications with which valproic acid is not recommended to be combined.
It is not recommended to take Depakine Chrono simultaneously with drugs that can provoke seizures or reduce the seizure threshold.
It is not recommended to combine the drug with Lamotrigine, as unwanted side effects may occur.
When combined with these drugs, strict medical supervision is required.
Analogues in composition
Pharmacies offer several complete analogues of the Depakine product. They have the same composition and chemical structure, but may differ in the volume of active substance.
Some contain auxiliary active ingredients that give the product additional healing properties.
Analogues of the product "Depakine" in composition:
- "Depakine Chrono" - the advantage of the product is prolonged absorption and the absence of a delay in absorption after administration. The active substance is released gradually, which avoids sudden changes in the concentration of the composition in the patient’s blood. The indicator remains stable for the entire duration of therapy. Unlike the classic analogue, it is prohibited for children under 6 years of age. In this case, the patient’s weight should not be less than 17 kg;
- "Depakine Enteric 300" is a broad-spectrum antiepileptic drug. The product is approved for use in pediatrics if the child is 3 years old;
- "Valproate Orion" - the composition can be used as an independent medicine or complement combination therapy. The tablets are film-coated. Thanks to this, the medication is characterized by a slow release of the active substance, which provides it with a long-term effect. Also, taking the drug allows you to maintain a uniform concentration of valproate in the blood, avoiding its fluctuations;
- "Encorat Chrono" is another analogue of the product "Depakine", which is characterized by prolonged absorption. It is enough to take it 1-2 times a day to achieve a therapeutic effect. In this case, differences in the concentration of the composition in the blood will be insignificant. An additional advantage of the product is its low price compared to other substitutes.

The product is approved for use in pediatrics if the child is over 3 years old.
It is prohibited to independently replace “Depakine” with any of the given analogues. Such decisions must be made by the doctor, taking into account the specifics of the situation, the goals of therapy, and the presence of additional factors.
Reviews
My three-month-old son was diagnosed with epilepsy. Childbirth was difficult, and during pregnancy I had a car accident.
I don’t know the exact reason; we are now going through the first stage of treatment. The doctor recommended Depakine in syrup, taken twice a day throughout the day.
A week after the start of therapy, the baby became calmer, there were no seizures yet, and his night sleep improved. I can't say anything bad about the drug.
Six months ago, I was repairing my neighbor’s roof when I accidentally fell and suffered a head injury. My epilepsy, which had previously been silent, became more active after the concussion. There were 4 attacks a day.
He underwent inpatient treatment, after which he was prescribed Depakin Chronosphere tablets. In the third week of taking the medicine, my state of health stabilized, irritability and unreasonable agitation disappeared.
Reviews of Depakine Chrono are usually positive. Patients take 300 mg and 500 mg tablets. Regardless of the form of release, the effectiveness of the drug is noted. However, almost every forum has reports of side effects when taking this drug. This indicates that the drug should be taken under strict medical supervision, carefully monitoring dosages.
Precautionary measures
Some problems may arise when using Depakine.
To avoid them, it is recommended to adhere to the following precautions:
- It is important to undergo tests periodically to monitor the activity of liver transaminases, platelets in the blood, bilirubin levels, etc. Studies are carried out every 2-3 months, especially when combining the drug with other pharmacological agents of similar action.

- The daily dosage changes throughout the therapy: at the initial stage, a minimum amount of the drug is supposed to be taken, then the dosage is increased weekly to the maximum determined by the attending physician. A couple of weeks before stopping the medication, the daily dose is reduced to the initial level.
- You cannot combine Depakine with drinking alcohol.

- If surgery is planned during the period of antiepileptic therapy or after its completion, the patient undergoes tests to monitor important indicators.
- If signs of an “acute abdomen” appear, you need to donate blood for testing. This will exclude or confirm the diagnosis of acute pancreatitis (monitoring amylase levels).

- If the patient has diabetes, the results of blood and urine tests may be distorted due to the concentration of keto products.
- You should not abruptly stop taking Depakine due to the high risk of increased frequency of epileptic seizures.
- Taking antiepileptic medications provokes a decrease in concentration and causes inhibition of psychomotor reactions. During the period of use of the medicine, driving motor vehicles and performing high-risk work should be avoided.
- To prevent the manifestation of dyspeptic disorders, it is recommended to include enveloping agents and antispasmodics in therapy.
Doctors' recommendations for selecting an analogue
Epilepsy is a serious and dangerous disease, which is treated with aggressive medications. Most of them are sold in pharmacies by prescription, which does not allow consumers to independently replace the products prescribed by a specialist with their analogues.
Neurologists warn against trying to purchase cheaper or hyped products online.
Such experiments often end in failure due to counterfeit or inappropriate drugs entering the patient’s body.
Pharmacies offer dozens of effective synonyms and analogues of Depakine. Many of them are presented in several dosage forms, which facilitates the selection of the drug and makes it possible to treat epilepsy at any age.

Pharmacies offer dozens of effective analogues of the drug.