Medicinal properties
This is a nootropic medicine. Under the influence of Cortexin, the functioning of the brain improves, and attention also increases. When using this medicine, a person becomes more susceptible to stressful situations and psycho-emotional stress.
Frequent use of this medication has an antioxidant effect on the body, and also protects cells from the effects of free radicals, strengthens the resistance of nerve cells in stressful situations and oxygen starvation. What is "Cortexin" for?
Indications and contraindications
The drug is prescribed to patients when a number of pathological processes occur:
- Traumatic brain injuries.
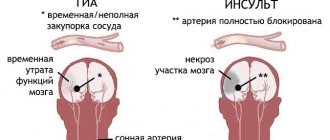
- Previous hemorrhagic strokes (acute cerebrovascular accident with vascular rupture and hemorrhage in the brain).
- The phenomena of cerebral ischemia (a complex pathology in which the blood vessels of the brain narrow, which impairs the flow of blood to the brain, and accordingly causes a lack of oxygen in the brain tissues).
- Encephalopathies (a general name for pathological processes of various origins, the basis of which is the degeneration of brain neurons due to disruption of their metabolism).
- Memory impairment.
For what diseases is Cortexin still used? The drug is prescribed for the following conditions:
- Decreased attention.
- Absent-mindedness.
- Forgetfulness.
- Epilepsy (a chronic neurological disease manifested in the body’s predisposition to the sudden onset of seizures).
- Poor performance.
- Failure to perceive new information.
- Vegetative-vascular dystonia with panic attacks (a polyetiological syndrome characterized by dysfunction of the autonomic nervous system).
- Birth injuries in newborns.
- Cerebral palsy (the result of brain damage received during pregnancy, childbirth and during the first 28 days of the baby’s life).
The drug can be used by patients only on the recommendation of a doctor after examination. The main contraindication for Cortexin is individual intolerance and increased sensitivity, pregnancy.
Peptide bioregulators and their application: from neonatology to gerontology
The peptide drug Cortexin, which is a complex of natural low-molecular compounds from the brain of animals (a highly purified extract of nuclear proteins), continues to be one of the most widely discussed pharmacological agents in periodical medical press. At the same time, two aspects attract attention: firstly, the use of Cortexin by representatives of various medical specialties (multidisciplinary in the intended use of the drug), and secondly, the absence of age restrictions on its use. In the modern pharmacopoeia there are not too many similar drugs that do not have age-related contraindications and restrictions on use. Therefore, Cortexin, rightly called the “drug of the 21st century,” deserves more detailed consideration [1].
Categorization and composition of Cortexin
As you know, Cortexin belongs to the pharmacological group 9.7 (“Nootropics (neurometabolic stimulants)”). According to the ATC system (anatomical-therapeutic-chemical) classification, the drug belongs to the heading N06BX (“Other psychostimulants and nootropic drugs”). Among the x-class peptide drugs (cytogens, cytamins, cytomedins), Cortexin belongs to the cytomedins, which are a highly purified extract of nuclear proteins (fractions 1–10 kDa) and are characterized by a proven property of regulating immunological reactivity [2]. Cortexin is an innovative peptide drug; its substance and dosage forms are protected by patents of the Russian Federation (RF No. 2104702, 2275924, 2195297) and other countries.
Cortexin is a multicomponent drug, the composition of which is not limited to neuropeptide substances. In addition to neuropeptides, Cortexin ingredients are represented by no less than three groups of substances: amino acids, vitamins and minerals. There is reason to believe that the positive effects of the drug are explained not only by the action of amino acids and polypeptides, but also by the neurochemical activity of macro- and microelements and vitamins [3, 4].
In particular, Cortexin peptides contain at least two stimulating amino acids - aspartic acid (446 nmol/mg) and glycine (298 nmol/mg). In addition to the above-mentioned aspartic acid and glycine, Cortexin contains the following amino acids:
- threonine (212 nmol/mg);
- serine (268 nmol/mg);
- glutamic acid (581 nmol/mg);
- proline (187 nmol/mg);
- alanine (346 nmol/mg);
- valine (240 nmol/mg);
- isoleucine (356 nmol/mg);
- tyrosine (109 nmol/mg);
- phenylalanine (162 nmol/mg);
- histidine (116 nmol/mg);
- lysine (253 nmol/mg);
- arginine and other amino acids (202 nmol/mg).
From the information presented it follows that aspartic acid accounts for up to 12%, and glutamic acid - about 15% of the total amino acid content in Cortexin peptides. The drug does not contain methionine. In addition, the stabilizer of the drug is the free amino acid glycine (12 mg), the role of which in normalizing the functions of the cerebral cortex has been proven many times.
Cortexin contains a number of vitamins, in particular water-soluble ones: thiamine (vitamin B1) - 0.08 mcg/10 mg, riboflavin (vitamin B2) - 0.03 mcg/10 mg, niacin (vitamin B3, vitamin PP, nicotinic acid) - 0.05 mcg/10 mg; as well as fat-soluble: retinol (vitamin A) - 0.011 mcg/10 mg, alpha-tocopherol (vitamin E) - 0.007 mcg/10 mg. We must admit frankly that this vitamin composition is optimal for the neurons of the brain, as it is perfectly balanced by nature!
In addition to the five essential vitamins, Cortexin contains many minerals (macro- and microelements):
- copper (Cu): 0.2129 µg/10 mg;
- iron (Fe): 2.26 µg/10 mg;
- Calcium (Ca): 22.93 µg/10 mg;
- magnesium (Mg): 8.5 µg/10 mg;
- potassium (K): 19.83 µg/10 mg;
- sodium (Na): 643.2 µg/10 mg;
- sulfur (S): 152.65 µg/10 mg;
- phosphorus (P): 91.95 µg/10 mg;
- zinc (Zn): 4.73 µg/10 mg;
- molybdenum (Mb): 0.0203 µg/10 mg;
- cobalt (Co): 0.0044 µg/10 mg;
- manganese (Mn): 0.0061 µg/10 mg;
- selenium (Se): 0.0745 µg/10 mg;
- aluminum (Al): 0.3104 µg/10 mg;
- lithium (Li): 0.0340 µg/10 mg [4].
Almost all of the listed mineral substances that make up the Cortexin drug have neuromodulatory and other functions.
Traditional and new areas of application of Cortexin
Cortexin is used not only in the Russian Federation. It is registered and used in countries such as Ukraine, Belarus, Armenia, Uzbekistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Tajikistan and Moldova (the use of Cortexin in all cases was preceded by preclinical and clinical examination).
The most important property of Cortexin, which determines the areas of its use, is considered to be the correction of cognitive functions, but the cognitive-modulating effect of the drug is far from the only one. The nootropic, neurotrophic, anticonvulsant, immunomodulatory (immunoregulatory) and neuroprotective effects of the drug, as well as its anti-stress, antioxidant and metabolic effects are described. In connection with the latter, I would like to especially note Cortexin’s ability to normalize the metabolism of neurotransmitters and regulate the balance of activating/inhibitory amino acids.
Therefore, over the past 10 years, Cortexin has been actively used by Russian doctors in various fields of clinical medicine (neurology, pediatrics, ophthalmology, gerontology, etc.): for traumatic brain injuries, neuroinfections, cerebral palsy (CP), perinatal nerve damage systems, disorders (delay) of psychomotor and speech development, thinking disorders, reduced learning ability, neurotic disorders, attention deficit hyperactivity disorder (ADHD), burnout syndrome, autonomic dysfunction, hypertensive angioretinopathy, glaucoma, diabetic neuropathy, amblyopia, etc. d. [1–6].
It should be noted that new areas of application are being discovered for the drug Cortexin. Thus, Bochkova L. G. and Nosova O. M. (2008) report on the experience of using Cortexin in the treatment of natal cervical injury in newborns, Kamenskikh T. G. et al. (2006) - in the treatment of partial optic nerve atrophy (PANA), Utaganova G. Kh. (2010) - in the correction of cervical spondylogenic lesions in young children [7–9].
Cortexin is actively used in gerontology (cerebrovascular accidents, asthenic conditions, encephalopathy of various origins, etc.) [10]. Suffice it to recall that the drug was first introduced into clinical practice by employees of the Military Medical Academy (on the battlefields in Afghanistan [16, 17]), and then by employees of the St. Petersburg Institute of Bioregulation and Gerontology of the Northwestern Branch of the Russian Academy of Medical Sciences. According to Corresponding Member of the Russian Academy of Medical Sciences, Prof. Khavinson V.Kh., who is the vice-president of the Gerontological Society of the Russian Academy of Sciences, “the use of peptide drugs makes it possible to effectively prevent premature aging, as well as treat diseases associated with age...”.
The use of Cortexin for migraine, recommended by Pak L.A. et al. (2005, 2006, 2008), Izzati-Zadeh K. F. et al. (2006), etc., has already become firmly established in the practice of Russian neurologists dealing with this group of primary cephalgia [11–14]. It is indicated that during a migraine attack, Cortexin stabilizes platelet membranes, inhibits the development of the “serotonin cascade,” suppresses neuronal cortical depression of Leo, reduces neurogenic inflammation of the vessels of the dura mater of the brain, suppresses the release of neuropeptides of pain and inflammation from perivascular afferent fibers of the trigeminal nerve [14].
Preliminary studies by the staff of the Scientific Center for Children's Diseases of the Russian Academy of Medical Sciences suggest that in the near future Cortexin will be used as a means for correcting various forms of neurological deficits accompanying certain types of food intolerance (celiac disease, lactase deficiency, etc.) in childhood, and subsequently in adult patients. But perhaps most impressive is the drug's potential use in the treatment of epilepsy.
Cortexin and epilepsy
Epilepsy therapy is a relatively recent indication for the use of Cortexin. The use of Cortexin in the complex treatment of various forms of epilepsy is reported in the works of Guzeva V. I. and Trubacheva A. N. (2003), Golovkin V. I. (2005, 2006) and Zvonkova N. G. (2006) [15–18 ]. Khorshev S.K. et al. (2002, 2008) consider Cortexin as a corrector of the neuroimmune component of epileptogenesis and, based on data from their own biochemical and neuroimmunophysiological studies, recommend this neuropeptide drug for the preventive treatment of epilepsy [19, 20]. The effectiveness of Cortexin in the treatment of epilepsy in children and adults is discussed in the publications of Tsygan V.N. et al. (2008) and Fedunova G.V. et al. (2008) [21, 22]. The need for correction of cognitive impairment with nootropic drugs for epilepsy is indicated by S. V. Balkanskaya et al. (2007, 2008) and Kalinin V.V. et al. (2009) [23–25]. It is Cortexin that plays an important role in this regard.
Finally, in April 2010, at the XVII Russian National Congress “Man and Medicine”, with the support, a symposium “Neuroprotection in the treatment of epilepsy” was held (chaired by Prof. A. B. Gekht). During the work of this symposium, the speakers (Kalinin V.V., Kalacheva I.O., Odintsova G.V.) reflected both theoretical aspects and their own experience of using the drug Cortexin in the treatment of epilepsy in children and adult patients.
In particular, at the Brain Institute of the Russian Academy of Sciences (N. Yu. Koroleva and G. V. Odintsova) there is a positive experience of using Cortexin in 8,000 patients, and, as A. B. Gekht noted, this institution receives “far from the mildest patients "
It is possible that in the future Cortexin will find wide use in the preventive treatment of febrile seizures (FS), which is the most common chronic disorder of cerebral functions in children.
Cortexin for children - a new form of neuropeptide drug
A new form of the drug called “Cortexin for Children” was registered in the Russian Federation on April 27, 2009 [26].
Cortexin for children is a lyophilisate for preparing a solution for intramuscular administration. 1 bottle (3 ml capacity) contains 5 mg of Cortexin (a complex of water-soluble polypeptide fractions with a molecular weight of not more than 10,000 Da) instead of 10 mg present in the standard form of the drug Cortexin, as well as 6 mg of glycine as a stabilizer.
In fact, Cortexin for children is almost the only example where a nootropic drug is targeted specifically for pediatric patients. It is expected that the new form of the peptide drug (Cortexin for children) will be in demand and will find wide application both in Russia and abroad.
What the “Formular System” is silent about
Previously, we have repeatedly pointed out that in the “Formular System” of Russia there is not even a mention of Cortexin [2, 4, 6]. We have to admit that in the XI issue (2010) of this annually updated publication there is no information about Cortexin again [27]. This is all the more strange when even the Internet encyclopedia Wikipedia provides data on this “pharmacopoeial polypeptide bioregulator with biological activity.”
The composition of Cortexin in the “Register of Medicines” (2010) is presented as follows: “in 1 bottle Cortexin - 10 mg, glycine - 12 mg” (glycine present in the drug simultaneously acts as a stabilizer) [28]. In the Vidal Reference (2010), the composition of Cortexin is described even more succinctly: “a complex of polypeptide fractions isolated from the cerebral cortex of cattle and pigs - 10 mg” [29]. In fact, as stated above, the ingredients are much more numerous.
We will try to present information about Cortexin using a form similar to that typically used in the Federal Drug Administration Guidelines.
Cortexin. A peptide preparation representing a complex of low molecular weight peptides isolated from the cerebral cortex of cattle (calves) and pigs under 12 months of age. To isolate the drug, the acetic acid extraction method is used. In order to obtain a fraction of polypeptides with a molecular weight not exceeding 10,000 Da (10 kDa), the resulting extract is subjected to multi-stage purification and repeated filtration on special filters. Purification of the active substance of Cortexin ensures the infectious and antigenic safety of the drug (the absence of infectious agents, nucleic acids, amyloids, functionally active pro-oncogenes and other undesirable impurities).
Cortexin has pronounced metabolic activity: normalization of neurotransmitter metabolism; regulation of the balance of inhibitory/activating amino acids and levels of serotonin and dopamine; GABAergic action; antioxidant effect; normalization of bioelectrical activity (BEA) of the brain. Possessing a pronounced tissue-specific effect on the cells of the cerebral cortex, this neuropeptide drug has cerebroprotective, nootropic, neurotrophic), neurometabolic, stimulating, anti-stress, antioxidant, anticonvulsant and immunoregulatory effects.
Indications. Traumatic brain injury (TBI), cerebrovascular accidents (CVA - acute and chronic), neuroinfections (viral and bacterial), asthenic conditions, encephalopathies of various origins, encephalitis (acute and chronic) and encephalomyelitis, epilepsy, various forms of cerebral palsy, critical conditions newborns with perinatal damage to the nervous system (PPNS), disturbances of psychomotor and speech development, disturbances (decrease) in memory and thinking; disorders of other cognitive functions (CF), headaches of various origins, etc.
Contraindications. Individual intolerance to the drug, pregnancy, lactation.
Side effects. When used according to indications, no side effects of Cortexin were identified.
Interaction. Drug interactions between Cortexin and other drugs have not been described.
Doses and application. The drug is prescribed intramuscularly for children weighing up to 20 kg at a dose of 0.5 mg/kg, and for children weighing more than 20 kg at a dose of 10 mg. The duration of treatment with Cortexin is usually 10 days. If necessary, a repeat course is provided after 1–6 (usually 3–6) months.
The contents of 1 bottle are dissolved in 1–2 ml of 0.5% novocaine solution, water for injection or 0.9% isotonic NaCl solution.
Cortexin. Lyophilized powder (lyophilisate) or porous mass of white/white color with a yellowish tint - for the preparation of a solution for intramuscular administration (vial), 1 ml (1 ml = 10 mg).
Cortexin for children. Lyophilisate for the preparation of solution for intramuscular administration (vial), 3 ml (5 mg).
* * *
In the new English-language scientific journal The Open Neuropsychopharmacology Journal, published since 2008 in the Netherlands, at the end of 2009 a joint publication of Russian and Italian scientists appeared (Moscow State University named after M.V. Lomonosov, L'Istituto Superiore di Sanita, Rome), dedicated to the use of the drug Cortexin and its effect on cognitive functions and behavioral reactions (under experimental conditions).
In particular, Adriani W. et al. (2009) report a confirmed anxiolytic effect of Cortexin, while emphasizing the activity of the drug when used in small doses and the absence of adverse reactions when used for medicinal purposes [30]. Such publications indicate not only the effectiveness of the neuropeptide bioregulator Cortexin from the standpoint of evidence-based medicine, but also the international recognition of this Russian drug.
The tissue specificity and high bioavailability of Cortexin determine an ever-increasing range of possibilities for the use of this drug in the treatment of patients, from the neonatal period to old age.
The reality of today's pharmacology is that there is actually a very meager list of drugs approved for use in children, and in this regard, the high therapeutic properties of Cortexin make it the drug of choice.
Literature
- Dyakonov M. M. Cortexin is a drug of the 21st century. Treatment and prevention of brain diseases // Aqua Vitae. 2001. No. 3. P. 22–23.
- Studenikin V. M. Use of the drug Cortexin in neuropediatrics // Med. messenger 2006. No. 37 (380). P. 14.
- Shabalov N. P., Platonova T. N., Skoromets A. P. Cortexin in neuropediatrics. Method. rec. St. Petersburg 2006. 64 p.
- Studenikin V.M., Pak L.A., Shelkovsky V.I. et al. The use of cortexin in pediatric neurology: experience and prospects // Pharmateka. 2008. No. 14. pp. 23–29.
- Granstrem O.K., Sorokina E.G., Storozhevykh T.P. et al. Latest news about cortexin (neuroprotection at the molecular level) // Terra Medica Nova. 2008. No. 5. P. 1–4.
- Studenikin V.M., Pak L.A., Shelkovsky V.I. et al. About the experience and prospects of using a domestic neuropeptide drug in pediatric neurology // Lech. Doctor. 2009. No. 5. pp. 42–45.
- Bochkova L. G., Nosova O. M. Nootropic and neuroprotective therapy for newborns with natal cervical trauma // Perinatology and Pediatrics. 2008. No. 1. P. 32–34.
- Kamenskikh T. G., Bashkatov A. N., Tuchin V. V. et al. Clinical and experimental rationale for the use of the drug “Cortexin” in the treatment of partial optic nerve atrophy // Russian Med. and. 2006. No. 4. pp. 147–150.
- Utaganova G. Kh. Natal cervical spondylogenic lesions in young children (clinic, diagnosis, treatment). Author's abstract. dis. ...cand. honey. Sci. M., 2010. 28 p.
- Morozov V. G., Khavinson V. Kh. Prospects for the use of cytomedins in clinical medicine and gerontology // Klin. gerontology. 2000. T. 78. No. 2. P. 42–45.
- Pak L. A., Goryunova A. V., Studenikin V. M. et al. Evaluation of the effectiveness of treatment of primary headaches in children with the peptide bioregulator Cortexin // Pediatrician. pharmacology. 2005. App. P. 121.
- Pak L. A., Goryunova A. V., Studenikin V. M. et al. Experience of clinical use of the drugs topiramate and cortexin in the preventive therapy of migraine in children // Issues. modern pediatrics. 2006. T. 5. No. 1. P. 441.
- Pak L. A., Goryunova A. V., Studenikin V. M. et al. Experience in the treatment of primary headaches in children // Doctor.ru. 2008. No. 4. pp. 28–30.
- Izzatizade K.F., Lodochnikova L.N., Shutov A.A. Migraine is another target for treatment with cortexin // Neuroimmunology. 2006. T. 4. No. 3–4. pp. 63–70.
- Guzeva V.I., Trubacheva A.N. Use of cortexin in the complex treatment of epilepsy in children // Terra Medica. 2003. No. 2. P. 19–21.
- Golovkin V.I. Cortexin in the treatment of epilepsy. In the book: Cortexin - five years of experience in domestic neurology / Ed. Skoromtsa A. A., Dyakonova M. M. St. Petersburg: Science. 2005. pp. 107–113.
- Golovkin V.I. Cortexin in the treatment of epilepsy // Medico-pharmacist. Vestn. Tatarstan. 2006. No. 6 (234). P. 15.
- Zvonkova N. G. Immunological parameters in children with epilepsy using traditional and alternative methods of therapy. Author's abstract. dis. ... Ph.D. M., 2006. 26 p.
- Khorshev S.K., Polyakov Yu.I., Bessmeltsev S.S. Cortexin as a corrector of the neuroimmune component of epileptogenesis. Mat. XI All-Russian. conf. "Neuroimmunology". St. Petersburg 2002. pp. 301–302.
- Khorshev S. L., Korsakova E. A., Stolyarov I. D. et al. Preventive treatment of epilepsy: the possibilities of cortexin (neuroimmunophysiological and biochemical study) // Neuroimmunology. 2008. T. VI. No. 1. pp. 22–26.
- Tsygan V.N., Mirolyubov A.V., Bogoslovsky M.M. et al. Efficacy of Cortexin in the treatment of epilepsy // Terra Medica Nova. 2008. No. 4. pp. 20–24.
- Fedunova G.V., Sysoeva E.N. Experience of using cortexin for symptomatic epilepsy in children // Chapter. doctor. 2008. No. 4 (16). P. 32.
- Balkanskaya S.V., Studenikin V.M., Kuzenkova L.M. et al. Nootropic drugs in the correction of cognitive functions in children with epilepsy // Issues. modern pediatrics. 2007. T. 6. No. 2. P. 92–96.
- Balkanskaya S.V., Studenikin V.M., Kuzenkova L.M. et al. Cognitive impairments and their correction in children with epilepsy // Pediatric Practice. 2008. No. 3. pp. 24–27.
- Studenikin V.M. Cortexin for children - a new form of a popular neuropeptide drug // Med. messenger 2009. No. 24. P. 13.
- Kalinin V.V., Zheleznova E.V., Sokolova L.V. et al. Cognitive and psychotropic effects of the drug Cortexin in the treatment of patients with epilepsy // Psychiatry and psychopharmacotherapy. 2009. T. 11. No. 3. P. 50–54.
- Federal guidelines for the use of medicines (formulary system). Vol. XI. M.: Echo. 2010.
- Register of Medicines of Russia "Encyclopedia of Medicines". 18th edition, revised. and additional M.: RLS-2010.
- Vidal Directory. Medicines in Russia: Directory. Ed. 16th, revised, corrected. and additional M.: AstraPharmService. 2010.
- Adriani W., Granstrem O., Romano E. et al. Modulatory effects of cortex and cortagen on locomotor activity and anxiety-related behavior in mice // Open Neuropsychopharmacology Journal. 2009. Vol. 2. P. 22–29.
V. M. Studenikin , Doctor of Medical Sciences, Professor L. A. Pak , Candidate of Medical Sciences S. Sh. Tursunkhuzhaeva V. I. Shelkovsky , Candidate of Medical Sciences S. V. Balkanskaya , Candidate of Medical Sciences
SCCD RAMS, Moscow
Contact information for authors for correspondence
Buy an issue with this article in pdf
How to use the medicine
According to the annotation, Cortexin is prescribed only for intramuscular injection.
The powder in the bottle is pre-dissolved in 1-2 milliliters of novocaine solution (0.5%), water for injections or isotonic sodium chloride solution.
The injection is given once a day, the dosage of the medicine is calculated by the doctor individually for each person, which depends on the weight, as well as the severity of the disorders and characteristics.
The duration of treatment should be at least 10 days; if necessary, therapy can be repeated after 3 months.
For people with stroke, a second course of treatment is carried out 10 days after the end of the previous one.
Method of therapy
It is very important to know how to take the medicine. It is produced in several pharmacological forms, depending on which the dosage of the drug is determined. Among them:
Solution for injection (4% 1 ml contains 40 mg of active ingredients). It is placed in glass ampoules and packaged in a cardboard box. These ampoules come in 2 ml, 5 ml and 10 ml. This medication is already ready for use.
As a rule, Actovegin injections can be used intravenously or intramuscularly. A single dosage (from 2 to 10 ml) is prescribed by the attending physician. To begin with, slowly inject 5 ml, increase the dose if necessary. Apply only 1 time per day.
- Solution for infusion. A dropper is used to administer it. This solution can be 10% (contains 25 ml of hemodialysate) and 20% (50 ml) per 250 ml of the finished solution. It also contains either sodium chloride or dextrose. Using a dropper, infuse a dose of solution at a rate of 2 ml per second. The therapeutic course is 10 infusions.
- Pharmacological forms for external use. Thus, ointment and cream contain 5 ml of hemodialysate, gel - 20 ml per 100 ml of total volume. The ointment and cream are a white homogeneous mixture, and the gel is a transparent or yellowish mass. Local preparations of Actovegin are applied in a thin layer to the affected area, which, if necessary, is covered with a sterile bandage.
Eye gel (20%). It is used in ophthalmology to treat eye wounds. To do this, squeeze a drop of gel directly into the eye. The procedure must be repeated three times a day.
- Actovegin tablets. They are prescribed for oral administration, 1-2 pieces at a time, three times a day before eating. The medicine should be taken without chewing, with liquid.
The tablets are yellow-green in color and oval in shape. They contain 200 mg of dry concentrate, as well as additional wax, cellulose, sucrose, talc, dye and other chemicals.
When prescribing one form or another of Actovegin, the doctor must take into account the excipients, since they can provoke an allergic reaction in the patient. Therefore, before taking Actovegin, you should ask a medical professional for advice.
For children
As is already known, Cortexin has no serious contraindications. Therefore, according to the annotation, the drug is allowed to be used by young patients from the first days of life.
The opinions of medical experts confirm the manufacturer’s statements that the medication causes almost no side effects.
The use of Cortexin in neurology, as well as neonatology and pediatrics, helps improve the child’s behavior, normalize memory and speech, and eliminate headaches.
The Ministry of Health canceled the registration of Actovegin and 41 drugs
Cancellation of registration of "Actovegin"
The Ministry of Health canceled the registration of four dosage forms of Actovegin:
- solution for infusion (in dextose solution), registration certificate: P N014635/02 dated March 14, 2008.
- cream, registration certificate: P N014635/05 dated March 14, 2008.
- solution for infusion (in sodium chloride solution), registration certificate: P N014635/01 dated February 26, 2008.
- ointments, registration certificate: P N014635/06 dated February 29, 2008.
The decision was made based on the application. In 2020, at the initiative of the company, the registration of Actovegin in gel form was canceled. According to the state register, Actovegin tablets and injection solution remain in circulation, registration certificates P N014635/03 and P N014635/04.
According to AlphaRM, in 2020 more than 7 million packages of Actovegin were sold in Russia for a total of 6.1 billion rubles.
The drug is indicated for the treatment of the consequences of trophic disorders, inflammatory skin diseases, metabolic and vascular disorders of the brain and improving wound healing.
Cancellation of registration from August 1 to August 24, 2020
During this period, the decision to cancel registration was made in relation to another 41 drugs:
- “Cromoglin”, eye drops (P N015765/01 dated 06/09/2009), nasal spray (P N015765/02 dated 06/19/2009), “Teva”.
- “Latisse”, drops (LSR-008174/10 dated 08/17/2010), “Allergan”.
- “Zimar”, eye drops (LP-001525 dated February 16, 2012), “Allergan”.
- “Optiv”, eye drops (LP-001549 dated February 29, 2012), “Allergan”.
- “Methyluracil-AKOS”, ointment (P N002909/01 dated July 21, 2008, “Synthesis”.
- “Erythromycin”, tablets (P N000139/01 dated 12/05/2007), “Synthesis”.
- "Diclofenac", tablets (LS-000821 dated 08/13/2010), "Synthesis".
- “Gentamicin”, powder for solution preparation (LS-001598 dated 08/04/2011), “Synthesis”.
- "Drotaverine", solution (LP-003415 dated January 18, 2016), "Synthesis".
- "Galmanin", powder for external use (LS-001366 dated July 18, 2011), "Synthesis".
- “Piperazine adipate”, tablets (P N002490/01 dated November 25, 2009), “Synthesis”.
- "Ernaf", hemopreservative solution (LSR-001571/08 dated March 14, 2008), "Sintez".
- “Gliclazide-AKOS”, tablets (P N002377/01 dated November 24, 2008), “Synthesis”.
- "Butorphanol", solution (LS-000703 dated 07/07/2010), "Synthesis".
- “Pilotimol MINI”, eye drops, (LSR-002554/07 dated 09/04/2007), “Synthesis”.
- “Nitroxoline”, tablets (P N001588/01 dated June 10, 2008), “Synthesis”.
- “Pilocarpine prolong”, eye drops (LP-000939 dated 10/18/2011), “Synthesis”.
- “Ofloxacin”, eye ointment (LSR-000028/08 dated January 16, 2008), “Synthesis”.
- "Imibact", solution (LP-003610 dated 05/12/2016), "Synthesis".
- “Vaseline”, ointment (P N002360/01 dated December 15, 2008), “Synthesis”.
- “Nitsergoline”, lyophilisate (LP-001811 dated 08/27/2012), tablets (P N003157/01 dated 10/28/2009), “Synthesis”.
- “Neohemodez”, solution (P N003499/01 dated December 15, 2008), “Synthesis”.
- "Lidocaine", solution (LP-003673 dated June 14, 2016), "Synthesis".
- "Clomegel", vaginal gel (LSR-006240/10 dated 07/01/2010), "Synthesis".
- “Corvalol”, drops (P N000198/01 dated 02/22/2011), “Synthesis”.
- “Levomycetin sodium succinate”, powder for preparing a solution (P N001174/01 dated November 19, 2007), “Synthesis”.
- “Ethambusin”, tablets (P N000809/01-2001 dated 04/13/2010), “Synthesis”.
- "Magnesium sulfate, solution (LP-003835 dated September 14, 2016), "Synthesis".
- “Calcium chloride”, solution (P N003975/01 dated January 18, 2010), “Synthesis”.
- “Rinorus”, nasal gel (LP-003830 dated September 14, 2016), “Synthesis.
- “Cefat”, powder for preparing a solution (P N001515/01-2002 dated June 10, 2008), “Synthesis”.
- "Potassium chloride", concentrate (LP-003214 dated September 23, 2015), "Synthesis".
- “Imibakt”, ointment (LP-004639 dated January 17, 2018), “Synthesis”.
- "Calciumfolinate-Ebewe", solution (P N014892/03 dated 04/01/2008), "Sandoz".
- “Salicylic-zinc paste”, paste (P N002896/01 dated July 22, 2008), “Synthesis”.
- "Oxamp", capsules (P N002258/01 dated 10/22/2008), "Synthesis".
- "Metronidazole", vaginal gel (LSR-000796/09 dated 02/06/2009), "Synthesis".
- “Sodium thiosulfate”, solution (LP-003450 dated 02/09/2016), “Synthesis”.
- “Mykoket”, ointment (LS-002463 dated February 14, 2011), “Synthesis”.
- "Cefalexin", powder for the preparation of suspension (P N000563/01 dated 04/09/2013), "Synthesis".
- “Phenoxymethylpenicillin”, powder for the preparation of suspension (LS-000491 dated 05/11/2010), tablets (LS-000139 dated 02/11/2010), “Synthesis”.
The decision to withdraw from the market was made based on statements from manufacturers or marketing authorization holders.
Suspension and restoration of circulation from August 1 to August 24, 2020
The Ministry of Health, based on letters from the Ministry of Industry and Trade, suspended the circulation of three drugs from August 3, 2020:
- Syrup "Kashnol" (P N014296/01 dated 02/12/2010), India.
- Cream "Clotrimazole" (P N013448/02 dated 08/31/2010), India.
- Tablets "Migrenol" (P N014953/01 dated July 18, 2008), USA.
At the same time, circulation of two drugs was resumed:
- From August 19, Ketoaminol tablets (LP-002756 dated December 15, 2014), JSC Pharm.
- From August 5, “Emla” patch (P N011054 dated 08/29/2008), “Emla” cream (P N014033/01 dated 12/23/2008), Ireland.
Lactation and pregnancy
A contraindication to Cortexin is pregnancy; the medication is prohibited during this period of time due to the lack of clinical experience and information regarding the safety of the effect on the intrauterine development of the fetus.
If medication therapy must be carried out during breastfeeding, the woman is advised to stop it.
Adviсe
"Cortexin" is used only on the recommendation of a doctor. The bottle with the dissolved medicine must be used immediately; if the medicine still remains, then for the next injection a new ampoule with powder is opened, and the previous one is thrown away.
If the patient has forgotten to give an injection, a double dosage of Cortexin cannot be administered; during the next injection, the usual concentration of the active substance is used. When carrying out the procedure, you must adhere to the rules of antiseptics so as not to provoke the occurrence of post-injection complications. Is it possible to take Cortexin for a cold?
It must be remembered that if the temperature rises, therapy with this drug is discontinued until recovery. This must be done to avoid complications. The same goes for colds.
This medication does not suppress the functioning of the central nervous system and does not inhibit the speed of psychomotor reactions, therefore it can be used for the treatment of vehicle drivers. The drug is dispensed with a prescription from a medical specialist.
Benefits of the drug
Cortexin is most effective for ailments such as traumatic brain injury, insufficient cerebral blood supply, asthenia, epilepsy and cognitive disorders. It is known that in people with VSD, due to unstable vascular function, osteochondrosis and frequent release of adrenaline, the brain does not receive enough blood, as a result of which the whole body has to suffer. This can be indicated, first of all, by symptoms such as headaches, surges in blood pressure, dizziness, arrhythmia, lack of energy and vigor, lethargy, and inability to work fully. It is in order to eliminate or reduce the severity of the listed manifestations that VSD sufferers are prescribed Cortexin. So, taking the drug helps:
- improving brain metabolism,
- accelerating the recovery of the body after exposure to certain negative factors;
- reducing the intensity of negative manifestations from taking psychotropics;
- improving attention and memory processes;
- stimulating mental activity.
In addition, taking Cortexin also helps to significantly increase stress resistance, which is exactly what all patients suffering from panic attacks need. If the psyche of the “alarmist” becomes less sensitive and vulnerable, the number of anxiety attacks will be significantly reduced, which means that psychological well-being will improve. When taking Cortexin for panic attacks, it is worth remembering that the maximum positive effect is achieved approximately 2 weeks after the start of treatment.
It is also worth noting that many VSD patients who intend to take Cortexin are afraid of such a side effect as pressure surges. However, the idea that this medication affects blood pressure has not been proven; the drug can be used for both hypertension and hypotension. This point is very important, since “alarmists” often suffer from high blood pressure, and they are prohibited from taking any drugs that provoke the occurrence of this symptom.
Substitutes for Cortexin
Similar in their pharmacological effects to the drug are:
- "Glycine".
- "Actovegin".
- "Neuroximet".
- "Nootropil"
- "Gingko biloba."
- "Piracetam."
- "Central-B".
- "Encephabol."
Before changing the drug to one of the above generics, it is important to consult a doctor, since these medications have different compositions, indications, and a number of contraindications.