Acetylcholine
This is the first neurotransmitter that scientists have discovered.
It is responsible for the transmission of impulses by motor neurons - and therefore for all human movements. In the central nervous system, the neurotransmitter takes on stabilizing functions: it brings the brain out of a state of rest when it is necessary to act, and vice versa, it inhibits the transmission of impulses when it is necessary to concentrate. In this it is helped by two types of receptors - accelerating nicotinic and inhibitory muscarinic. Acetylcholine plays an important role in learning and memory formation. This requires both the ability to focus attention (and inhibit the transmission of distracting impulses) and the ability to switch from one subject to another (and speed up the reaction). Active brain activity, such as when preparing for an exam or an annual report, leads to an increase in acetylcholine levels. If the brain is inactive for a long time, a special enzyme, acetylcholinesterase, destroys the neurotransmitter, and the effect of acetylcholine weakens. Ideal for studying, acetylcholine will be a poor assistant in stressful situations: it is a mediator of reflection, but not of decisive action.
An excess of acetylcholine in the body causes spasms of all muscles, convulsions and cessation of breathing - this is precisely the effect that some nerve gases are designed for. A lack of acetylcholine leads to the development of Alzheimer's disease and other types of senile dementia. As maintenance therapy, patients are prescribed a drug that blocks the destruction of acetylcholine - an acetylcholinesterase inhibitor.
The CHRNA3 gene encodes the nicotinic acetylcholine receptor, which can be affected by nicotine. At the first stage, the substance acts on the sympathetic system of the body, which is responsible for spasm of smooth muscles and contraction of blood vessels. Therefore, for novice smokers, cigarettes cause nausea and pale skin rather than delight. But over time, nicotine reaches brain cells and activates acetylcholine receptors. Since both nicotine and acetylcholine do this at the same time, the brain tries to correct the “double supply”, and after a while the brain neurons reduce the normal production of acetylcholine. From now on, the smoker will need nicotine for every reason - in the morning to cheer up, after a meeting, on the contrary, to calm down, after lunch - to think at least a little about the eternal.
Polymorphism of the CHRNA3 gene affects the rate of formation of nicotine addiction and, as a consequence, the risk of developing lung cancer caused by smoking.
Specifics of influence
Acetylcholine is not a selective element of the system. To one degree or another, it affects both m- and n-molecules. Of interest is the muscarinic-like effect that acetylcholine has. This effect is manifested in a slowdown of the heart rate, dilation of blood vessels (peripheral), activation of intestinal and stomach peristalsis, contraction of the muscles of the uterus, bronchi, bladder, gall bladder, intensification of secretion of the bronchial, sweat, digestive glands, and miosis.
Adenosine
All chemical reactions in the body require energy. The currency used in this process is an adenine molecule with several phosphoric acid bases. Immediately after your “salary” you will see “three hundred rubles” on your card - a molecule of adenosine triphosphate
with three phosphoric acid residues.
Each transaction costs one hundred rubles, respectively, after the first “purchase” there will be only two hundred rubles left on the account (adenosine diphosphate
), after the second - one hundred rubles (adenosine
monophosphate
), after the third - zero rubles.
A bill of zero rubles is adenosine. As a neurotransmitter, it is responsible for feeling tired and falling asleep. During sleep, threes are added to bills of zero-zero rubles, adenosine is transformed into adenosine triphosphate, and we are ready to return to work with renewed vigor.
There is a way to deceive the “banking system”: block adenosine receptors and go on credit. This is exactly what caffeine does - it allows you to ignore fatigue and continue working. At the same time, it does not bring real energy, but only allows you to spend money, as if you still have three hundred rubles. Like any loan, you have to pay for overspending - with greater fatigue, retardation of attention, and addiction. However, caffeinated coffee, tea and chocolate are the most popular stimulants in the world.
There are four known types of adenosine receptors, which are activated and blocked by adenosine. The ADORA2A gene encodes type 2 adenosine receptors, which are involved in the activation of anti-inflammatory processes, the formation of the immune response, the regulation of pain and sleep. The speed of the body’s response to injury and injury depends on the functioning of this receptor.
Physiology of nicotine molecules
The first description was facilitated by intracellular conduction of electrical potentials. The nicotinic receptor was one of the first to record currents passing through a single channel. In the open state, K+ and Na+ ions and, to a lesser extent, divalent cations can pass through it. In this case, the channel conductivity is expressed in a constant value. The duration of the open state, however, is a characteristic that depends on the potential voltage applied to the receptor. In this case, the latter stabilizes during the transition from membrane depolarization to hyperpolarization. In addition, the phenomenon of desensitization is noted. It occurs with prolonged application of acetylcholine and other antagonists, which reduces the sensitivity of the receptor and increases the duration of the open state of the channel.
Glutamate
Glutamic acid, in the form of glutamate, is a dietary amino acid found in animal products.
Taste buds perceive glutamate as an indicator of protein food - and therefore nutritious and healthy - and leave a note that it was tasty and should be repeated. In the twentieth century, Japanese scientists figured out the principle of perception of this taste (they called it “umami” - tasty), and over time, monosodium glutamate became a popular food additive. It is thanks to him that it is sometimes difficult to resist the temptation to eat doshirak noodles. As a food additive, glutamate does not directly affect the functioning of neurons, so an “overdose” of it, in the worst case, will result in a headache. Glutamate is not only a dietary amino acid, but also an important neurotransmitter, the receptors of which are present in 40% of neurons in the brain. It does not have its own “semantic load”, but only accelerates signal transmission by other receptors - dopamine, norepinephrine, serotonin, etc. This function allows glutamate to form synaptic plasticity - the ability of synapses to regulate their activity depending on the response of postsynaptic receptors. This mechanism underlies the process of learning and memory.
Decreased glutamate activity leads to lethargy and apathy. An excess leads to “overvoltage” of nerve cells and even their death, as if the electrical network was given a greater load than it can withstand. “Burnout” of neurons—excitotoxicity—is observed after attacks of epilepsy and in neurodegenerative diseases.
Two groups of genes encode glutamate transporter proteins. The EAAT group genes are responsible for sodium-dependent proteins - the same ones that are involved in the memory process. Mutations in the genes of this group increase the risk of stroke, Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis. Mutations in the genes of vesicular transporter proteins of the VGLUT group are associated with the risk of schizophrenia.
Gamma-aminobutyric acid
Each yin has its own yang, and glutamate has its eternal enemy, with which it is nevertheless inextricably linked.
We are talking about the main inhibitory neurotransmitter - gamma-aminobutyric acid (GABA or GABA). Like glutamate, GABA does not add new colors to the palette of brain activity, but only regulates the activity of other neurons. Like glutamate, GABA covers about 40% of brain neurons with its network of receptors. Both glutamate and GABA are synthesized from glutamic acid and are essentially extensions of each other. To describe the effect of GABA, the saying “the slower you go, the further you go” is ideal: the inhibitory effect of the neurotransmitter allows you to concentrate better. GABA reduces the activity of a wide variety of neurons, including those associated with feelings of fear or anxiety and those that distract from the main task. High concentrations of GABA promote calm and composure. A decrease in GABA concentration and an imbalance in the eternal resistance with glutamate leads to attention deficit disorder (ADHD). Walking, yoga, and meditation are good for increasing GABA levels; most stimulants are good for decreasing them.
Gamma-aminobutyric acid has two types of receptors - the fast-acting GABA-A and the slower-acting GABA-B. The GABRG2 gene encodes the GABA-A receptor protein, which sharply reduces the speed of impulse transmission in the brain. Mutations in the gene are associated with epilepsy and febrile seizures, which can occur with high fever.
If dopamine, serotonin and norepinephrine are Hollywood actors in the big neural film industry, then the heroes of the second part of the story about neurotransmitters rather work behind the scenes. But without their invisible contribution, great cinema would be completely different.
In the next part
Acetylcholine transmits nerve impulses at cholinergic synapses. The discovery of the mediator role of acetylcholine belongs to the Austrian pharmacologist O. Lewi. Cholinergic synapses are present in both the somatic and autonomic nervous systems. Motor fibers of the somatic nervous system innervate skeletal muscles, and acetylcholine is released from their endings. The efferent pathways of the autonomic nervous system consist of two neurons: the first is located in the central nervous system (in the brain stem and spinal cord), the second is in the autonomic ganglion, which belongs to the peripheral nervous system (Fig. 5). Accordingly, the processes of the first neurons form preganglionic fibers, and the second - postganglionic. In the preganglionic neurons of both the sympathetic and parasympathetic divisions of the autonomic nervous system, acetylcholine is the main transmitter. The sympathetic and parasympathetic divisions differ in the mediator released at the synapses of the postganglionic fiber: in the sympathetic nervous system it is norepinephrine, in the parasympathetic nervous system it is acetylcholine. Thus, acetylcholine serves as a transmitter of impulses from the endings of all parasympathetic postganglionic fibers, from the endings of postganglionic sympathetic fibers innervating the sweat glands, from the endings of all (both sympathetic and parasympathetic) preganglionic fibers, from the endings of the motor nerves of striated muscles, as well as in many central synapses.
Chemically, acetylcholine is an ester of choline and acetic acid. Its synthesis takes place in the endings of nerve fibers from choline alcohol and acetyl-CoA under the influence of the enzyme choline acetyltransferase. The rate of the synthesis reaction is limited by the concentration of choline in synaptic endings. The synthesized mediator is deposited in vesicles as a result of active transport with the participation of the enzyme Mg^-dependent ATPase. The main mechanism for the release of acetylcholine into the synaptic cleft, resulting in the formation of a postsynaptic potential, is Ca2+-dependent exocytosis. Depolarization of the nerve ending, which increases the permeability of the presynaptic membrane to Ca2+, is a necessary condition for the release of acetylcholine. Acetylcholine is chemically unstable; in an alkaline environment it quickly breaks down into choline and acetic acid. Its destruction in the cholinergic synapse is catalyzed by the enzyme acetylcholinesterase, discovered by O. Levy. Acetylcholinesterase is located on the postsynaptic membrane next to the cholinergic receptor and is one of the fastest-acting enzymes. The rapid destruction of the transmitter ensures the lability of cholinergic nerve transmission. The resulting choline is captured by transporter proteins of the presynaptic membrane and further serves to restore acetylcholine in the terminal (Fig. 6).
/>Fig. 6. Scheme of the structure of the cholinergic synapse (cited from: Markova I.N., Nezhentseva M.N., 1997): ACh - acetylcholine; XR - cholinergic receptor; M - muscarinic cholinergic receptor; N - nicotinic cholinergic receptor; AChE - acetylcholinesterase; TM—transport mechanism; CA - choline acetyltransferase; (+) — activation; (-) — braking
The effect of acetylcholine on the membrane consists of its reaction with cholinergic receptors included in the structure of the cell membrane (Fig. 7). Thus, the reaction of acetylcholine with the H-cholinergic receptor causes a change in the spatial arrangement of the atoms of the protein molecule of the receptor. As a result, the size of the intermolecular pores of the membrane increases, forming a free passage for Na+ and then K+ ions, and depolarization of the cell membrane occurs, followed by repolarization. Changes in the receptor molecule caused by acetylcholine are easily reversible. After the impulse is transmitted, depolarization ends within approximately 1 ms and normal membrane permeability is restored. By this time, the cholinergic receptor is already free from connection with acetylcholine. It is believed that the deformation of the receptor molecule caused by acetylcholine leads not only to an increase in the intermolecular pores of the membrane, but also contributes to the rejection of acetylcholine from the receptor. This rejection is necessary for the interaction of releasing acetylcholine with acetylcholinesterase and its subsequent destruction (see Fig. 7). Substances that affect cholinergic receptors can cause a stimulating (cholinomimetic) or inhibitory (cholinolytic) effect.
O. C-0-CH2CH2-N(CH3)3
/ C-0-CH2CH2-N(CH3)3 сн3 Fig. 7. Scheme of interaction of acetylcholine with the cholinergic receptor and acetylcholinesterase (cited from: Zakusov V.V., 1973): XR - cholinergic receptor; AChE - acetylcholinesterase; A - anodic center of ChR and AChE; E - esterase center of AChE and esterophilic center of ChR Pharmacological substances can affect the following stages of synaptic transmission of cholinergic synapses: synthesis of acetylcholine; 2) the process of mediator release; 3) interaction of acetylcholine with cholinergic receptors; 4) destruction of acetylcholine; 5) capture by the presynaptic terminal of choline formed during the destruction of acetylcholine. For example, botulinum toxin acts at the level of presynaptic terminals, preventing the release of the transmitter. Transport of choline across the presynaptic membrane (neuronal uptake) is inhibited by hemicholine. Cholinomimetics (pilocarpine, cytisine) and anticholinergics (M-cholinergic blockers, ganglion blockers and peripheral muscle relaxants) have a direct effect on cholinergic receptors. Anticholinesterase drugs (prozerin) can be used to inhibit the acetylcholinesterase enzyme.
N, N, N-trimethyl-2-aminoethanol acetate
Functions
The function of acetylcholine is to serve as a neurotransmitter within the CNS (central nervous system). This substance affects the transmission of impulses from one part of the brain to another. At the same time, a small content of this substance promotes the transmission of impulses, and a significant amount of it inhibits it.
Acetylcholine also serves to be transferred to the muscles of the body. With a lack of this substance, the force with which muscles contract decreases. The lack of this particular compound leads to the fact that a person begins to suffer from Alzheimer's disease.
The effect of acetylcholine is expressed in a slower heart rate, a decrease in blood pressure, and an increase in the diameter of peripheral blood vessels. The compound improves peristalsis in the digestive tract (intestines and stomach). Also, its presence enhances the contractility of the muscles of a number of organs, including the urinary and gall bladders, uterus, and bronchi. Acetylcholine enhances iron secretion, in particular in the lacrimal, sweat, bronchial and digestive glands.
In addition, it causes constriction of the pupil (miosis), this effect is the result of more intense contraction of the circular muscle that controls the iris, which is influenced by postganglionic cholinergic fibers located in the oculomotor nerve. This constriction of the pupil most often occurs in combination with a decrease in intraocular pressure. This is due to the fact that with such a narrowing there is an expansion of Schlemm’s canal, as well as the space in the corner formed by the iris and cornea. As a result, the fluid receives a greater opportunity for outflow from the internal environment of the eye.
Acetylcholine also serves to improve concentration by producing neurons located in.
Another function of the compound is its influence on falling asleep and waking up. The sleeper wakes up after the intensity of the activity of cholinergic neurons located in the brain stem, as well as in the forebrain in the basal ganglia, increases.
Acetylcholicin, produced artificially, is used for treatment only in some cases. This is due to the fact that when taken orally, this compound quickly undergoes hydrolysis, as a result of which its absorption from the mucous membranes of the gastrointestinal tract does not occur. When introduced into the body in any other way, including through injection, it also does not have a significant effect on the central nervous system. That is why now in most cases they refuse it.
It is also important to keep in mind that acetylcholine constricts the veins in the heart. If an excessive dose of this substance is administered to a patient, the result may be bradycardia, a drop in blood pressure, arrhythmia, sweating and other adverse effects.
Acetylcholine
- one of the most important neurotransmitters, it carries out neuromuscular transmission and is the main one in the parasympathetic nervous system.
It is destroyed by the enzyme acetylcholinesterase
.
It is used as a medicinal substance and in pharmacological studies.
Chemical properties
Acetylcholine is the main neurotransmitter
, responsible for neuromuscular transmission in the parasympathetic nervous system.
It is a quaternary monoammonium compound. The substance itself is not stable; it is quickly destroyed in the body by acetylcholinesterase
, resulting in the formation of
acetic acid
and
choline
.
The product is synthesized in the form of white crystals or a crystalline mass, which tends to diffuse upon contact with air. The substance dissolves well in alcohol and water. It cannot be boiled or stored for a long time, acetylcholine decomposes.
Used as a drug that improves neuromuscular transmission and for pharmacological studies. It is often synthesized as a salt or chloride
.
This neurotransmitter plays an important role in the body, increases brain performance and memory. Therefore, it is important that there is enough acetylcholine in the foods included in the daily diet.
Constriction of the pupil
The circular muscle of the iris, innervated by postganglionic fibers, begins to contract intensively simultaneously with the ciliary muscle. In this case, the ligament of cinnamon relaxes. As a result, a spasm of accommodation occurs. Constriction of the pupil associated with the influence of acetylcholine is usually accompanied by a decrease in intraocular pressure. This effect is partly due to the expansion of the membrane in Schlemm's canal and fountain spaces against the background of miosis and flattening of the iris. This helps improve the outflow of fluid from the internal ocular media.
Due to the ability to lower intraocular pressure, like acetylcholine, drugs based on other similar substances are used in the treatment of glaucoma. These include, in particular, cholinomimetics.
Pharmacodynamics and pharmacokinetics
The cholinomimetic effect of Acetylcholine on the body occurs due to its stimulation of n-
and
m-cholinergic receptors
. The substance slows down heart contractions, dilates peripheral blood vessels, reduces and enhances intestinal and stomach motility.
The drug affects the secretion of bronchial and digestive glands, the elimination of sweat and tears. The substance also produces a miotic effect, enhances (constriction of the pupil), and reduces.
Small doses of acetylcholine stimulate the transmission of nerve impulses in various parts of the brain, while large doses, on the contrary, inhibit this process. This neurotransmitter generally improves brain performance and memory. Therefore, it is important that there is enough acetylcholine in the foods included in the daily diet. With its deficiency, brain dysfunction develops ().
Nosological classification (ICD-10)
Some molecules are found in the region of cholinergic postganglionic nerves. This is the area of smooth muscles, heart, glands. They are called m-cholinergic receptors - muscarine-sensitive. Other proteins are located in the area of ganglion synapses and in neuromuscular somatic structures. They are called n-cholinergic receptors - nicotine-sensitive.
The nicotine-like effect of acetylcholine is determined by its participation in the process of transmitting signals from preganglionic nerve fibers to postganglionic nerve fibers located in the autonomic ganglia, and from motor endings to striated muscles. In small doses, the substance acts as a physiological transmitter of excitation.
- H34.0 Transient retinal arterial occlusion
- I70.2 Atherosclerosis of the arteries of the extremities
- N31.2 Neurogenic bladder weakness, not elsewhere classified
- Z100* CLASS XXII Surgical practice
Overdose
Overdose can cause a sharp decrease in blood pressure
,
bradycardia
, cardiac arrest, rhythm disturbances,
miosis
,
diarrhea
and so on.
To eliminate unwanted symptoms, it is recommended to administer 1 ml of 0.1% solution or other anticholinergic drug
(for example,
The product is sometimes included in some combinations. preparations for topical use in eye surgery to create persistent and long-lasting miosis
.
Sources
- Rockland, K. S. Brain. In A. E. Kazdin (Ed.), Encyclopedia of psychology (Vol. 1, pp. 447–455). Washington, DC: American Psychological Association.
: Incorrect or missing image To improve this article it is desirable: - Wikify the article.
- Rework the design in accordance with the rules for writing articles.
- Find and arrange in the form of footnotes links to independent authoritative sources that confirm what is written. K: Wikipedia: Articles without sources (type: not specified)
Properties
Physical
Colorless crystals or white crystalline mass. Dissolves in the air. Easily soluble in water and alcohol. When boiled and stored for a long time, the solutions decompose.
Medical
The physiological cholinomimetic effect of acetylcholine is due to its stimulation of the terminal membranes of M- and N-cholinergic receptors.
The peripheral muscarinic-like effect of acetylcholine manifests itself in a slowdown of heart contractions, expansion of peripheral blood vessels and a decrease in blood pressure, increased peristalsis of the stomach and intestines, contraction of the muscles of the bronchi, uterus, gall and bladder, increased secretion of the digestive, bronchial, sweat and lacrimal glands, miosis. The miotic effect is associated with increased contraction of the orbicularis iris muscle, which is innervated by postganglionic cholinergic fibers of the oculomotor nerve. At the same time, as a result of contraction of the ciliary muscle and relaxation of the zonular ligament of the ciliary girdle, a spasm of accommodation occurs.
Constriction of the pupil caused by the action of acetylcholine is usually accompanied by a decrease in intraocular pressure. This effect is partly explained by the fact that when the pupil narrows and the iris flattens, Schlemm's canal (venous sinus of the sclera) and fountain spaces (spaces of the iridocorneal angle) expand, which ensures better outflow of fluid from the internal media of the eye. It is possible that other mechanisms are also involved in the decrease in intraocular pressure. Due to their ability to reduce intraocular pressure, substances that act like acetylcholine (cholinomimetics, anticholinesterase drugs) are widely used for the treatment of glaucoma. It should be borne in mind that when these drugs are introduced into the conjunctival sac, they are absorbed into the blood and, having a resorptive effect, can cause side effects characteristic of these drugs. It should also be borne in mind that long-term (over a number of years) use of miotic substances can sometimes lead to the development of persistent (irreversible) miosis, the formation of posterior petechiae and other complications, and long-term use of anticholinesterase drugs as miotics can contribute to the development of cataracts.
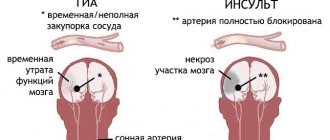
Acetylcholine also plays an important role as a central nervous system neurotransmitter. It is involved in the transmission of impulses in different parts of the brain, with small concentrations facilitating, and large concentrations inhibiting synaptic transmission. Changes in acetylcholine metabolism lead to severe disruption of brain function. Its deficiency largely determines the clinical picture of such a dangerous neurodegenerative disease as Alzheimer's disease []. Some centrally acting acetylcholine antagonists (see Amizil) are psychotropic drugs (see also Atropine). An overdose of acetylcholine antagonists can cause disturbances in higher nervous activity (have a hallucinogenic effect, etc.). The anticholinesterase effect of a number of poisons is based precisely on the ability to cause the accumulation of acetylcholine in synaptic clefts, overexcitation of cholinergic systems and more or less rapid death (chlorophos, karbofos, sarin, soman) (Burnazyan, “Toxicology for medical university students,” Kharkevich D.I., “ Pharmacology for students of the Faculty of Medicine").
Explanations
The above classification is determined by the specificity of the reactions that occur when these biochemical systems and acetylcholine interact. This, in turn, explains the reasons for some processes. For example, a decrease in pressure, increased secretion of gastric, salivary and other glands, bradycardia, constriction of the pupils, etc. when affecting muscarine-sensitive proteins and contraction of skeletal muscles, etc. when affecting nicotine-sensitive molecules. Moreover, recently scientists have begun to divide m-cholinergic receptors into subgroups. The role and localization of m1- and m2-molecules are the most studied today.
Application
General Application
Acetylcholine chloride (lat. Acetylcholini chloridum)
). Acetylcholine chloride is not widely used as a medicine.
Treatment
When taken orally, acetylcholine is hydrolyzed very quickly and is not absorbed from the mucous membranes of the gastrointestinal tract. When administered parenterally, it has a quick, sharp and short-lasting effect (like adrenaline). Like other quaternary compounds, acetylcholine penetrates poorly from the vascular bed through the blood-brain barrier and does not have a significant effect on the central nervous system when administered intravenously. Sometimes in experiments, acetylcholine is used as a vasodilator for spasms of peripheral vessels (endarteritis, intermittent claudication, trophic disorders in the stumps, etc.), and for spasms of the retinal arteries. In rare cases, acetylcholine was administered for intestinal and bladder atony. Acetylcholine has also sometimes been used to facilitate x-ray diagnosis of esophageal achalasia.
General information
The endings of the fibers from which the transmitter acetylcholine is transmitted are called cholinergic.
In addition, there are special elements with which it interacts. They are called cholinergic receptors. These elements are complex protein molecules - nucleoproteins. Acetylcholine receptors have a tetrameric structure. They are localized on the outer surface of the plasmatic (postsynaptic) membrane. By their nature, these molecules are heterogeneous. In experimental studies and for medical purposes, the drug “Acetylcholine chloride” is used, presented in an injection solution. No other medicines based on this substance are available. There are synonyms for the drug: “Myohol”, “Acecoline”, “Cytocholine”.