Glioblastoma of the brain is by far the most dangerous type of all malignant brain tumors, being the most frequently diagnosed primary tumor of the central nervous system. The main distinguishing feature of glioblastoma is the chaotic arrangement of cells in it, as well as deformation of the vascular bed, pronounced edema and the presence of areas of necrosis of brain tissue. In addition, the disease is characterized by rapid progression with the involvement of an increasing number of healthy areas in the process - which is why the tumor has no visible boundaries.
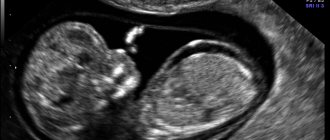
Glioblastoma on a tomogram
How many people live with this diagnosis, and what is the prognosis for future life after removal of glioblastoma? Unfortunately, the answers to these questions are disappointing.
What is glioblastoma
Glioblastoma of the brain is a malignant tumor, it is formed from astrocytes. As a rule, this type of brain tissue cancer is diagnosed in males and is localized in the cortex, frontal lobes or brain stem.
The neoplasm develops as a result of the uncontrolled proliferation of astrocytes - these are stellate glial cells. Every person should have an idea of what glioblastoma of the brain is in order to be able to consult a doctor in time.
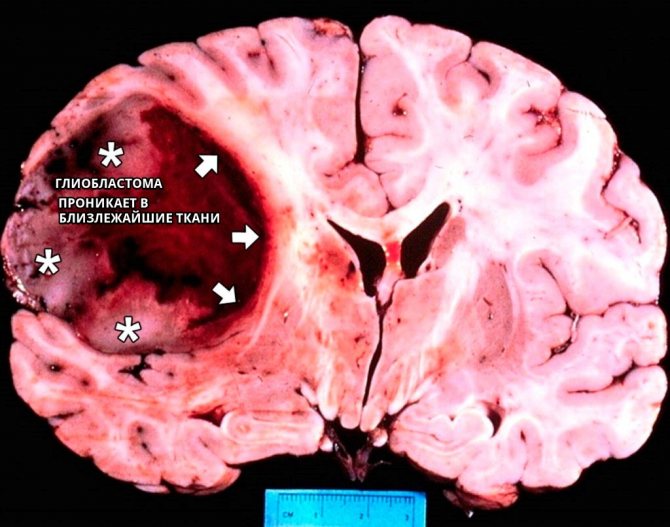
Glioblastomas are characterized by a chaotic accumulation of tumor cells with an increased number of nuclei, which are interspersed with foci of necrosis and altered blood vessels.
This type of malignant tumor that appears in the brain is very dangerous due to its rapid growth and lack of clear distinctions between affected and healthy tissues.
Forecast
The life expectancy of patients suffering from this disease is short and rarely exceeds five years. Even identifying a tumor in the early stages followed by radical treatment does not solve the problem, since the likelihood of relapse remains high.
Glioblastoma is one of the most malignant brain tumors. It is difficult to treat and can progress rapidly, leading to damage to vital centers. All this minimizes the patient's chances of survival.
Types of glioblastoma
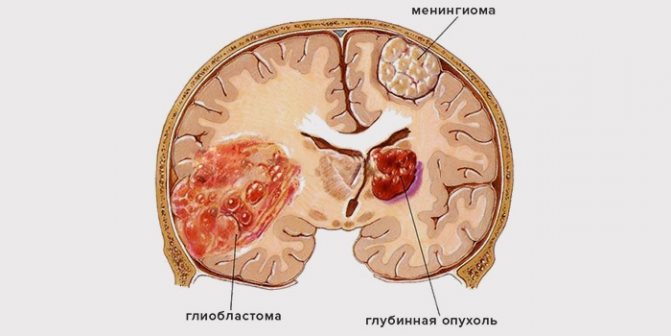
There are several types of this malignant neoplasm. It may differ in the degree of malignancy, determined by histological examination of tissue samples taken.
There are three types of tumor:
- Giant cell.
- Multiform.
- Gliosarcoma.
Diagnosis of a specific form is very important because it helps to choose the most effective treatment. Different types of oncology are unequally sensitive to chemotherapy and radiotherapy.
Giant cell glioblastoma
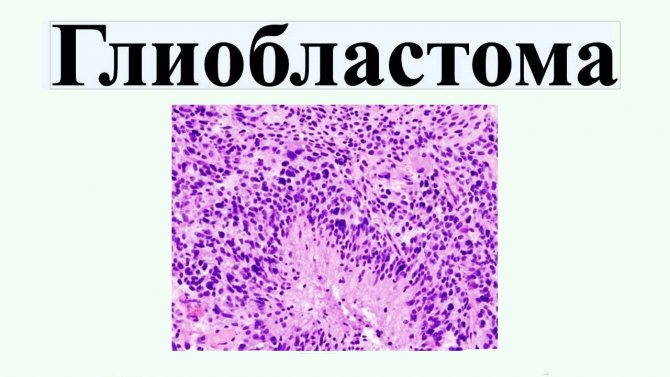
Giant cell glioblastoma is a type of tumor characterized by the presence of abnormally large cells with an increased number of nuclei.
Glioblastoma multiforme
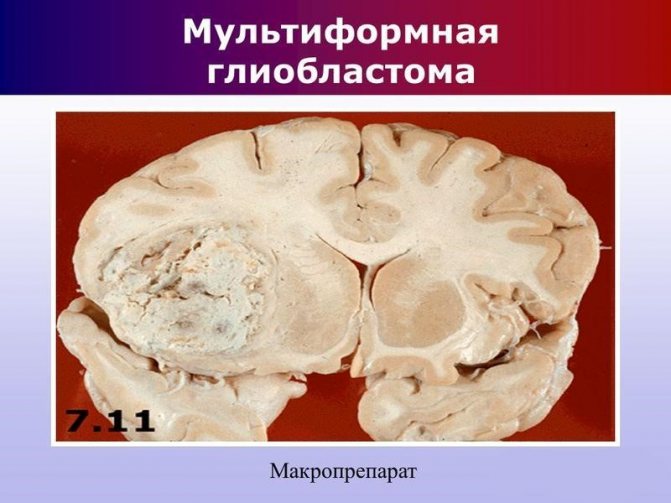
Glioblastoma multiforme is the most dangerous form with the presence of foci of necrosis. In addition to cell damage, there is a rapid progression of necrotic processes in the vessels. This provokes terrible and irreversible consequences.
Gliosarcoma
Gliosarcoma is the least common. This is a glioblastoma of the brain stem. If treatment is not timely, the opposite half of the head grows through the corpus callosum. This type is less dangerous than the previous one, but the chances of successful consequences and prognosis are reduced due to the inability to predict the course of the disease.
Preventive measures
Preventing brain cancer consists of following fairly simple rules:
- Be sure to get enough sleep, because... healthy sleep in normal quantities promotes brain recovery;
- limit the consumption of energy drinks and large amounts of coffee;
- rest for the required amount of time;
- don't worry, be less nervous.
- Don't eat processed pork;
- eat fresh vegetables and fruits in large quantities;
- overcome bad habits;
- use your mobile phone minimally, use a speakerphone or headset;
- give up smoking and sausages.
Brain cancer often develops in people between 50 and 65 years of age. Being at this age, you need to pay close attention to your health, and if necessary, undergo an examination using magnetic resonance imaging of the head.
This method will help detect any brain formation in the early stages and begin treatment on time.
Glioblastoma of the brain is an extremely serious disorder that often ends in death. But a timely diagnosis and surgery increases the chances of a normal life.
Causes of tumor development and risk factors
Any adult or child can suffer from cancer. The main reason is a hereditary predisposition to cancer. Also, the risk of cancer cells increases in the presence of congenital or acquired genetic changes. Risk factors that may lead to the disease also include:
- cytomegalovirus;
- radiation exposure;
- traumatic brain injuries;
- smoking.
Glioblastoma of the brain occurs spontaneously, but there are also familial cases - approximately 1% of all diagnosed. Scientists also claim that the use of pesticides may be a factor in the growth of malignant tumors, but this theory is still only an assumption.
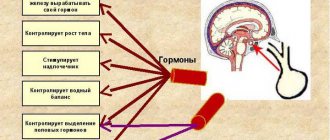
In women, the risk of development and growth of tumors most often increases during menopause, and therefore a theory has emerged about the influence of hormones on the occurrence of tumors in brain cells.
Symptoms
Glioblastoma or grade 4 brain cancer according to the international classification of diseases ICD 10 is coded C71. Next, depending on the location of the neoplasm, a numerical value follows:
- 0 – final section;
- 1 – frontal lobe;
- 2 – temporal;
- 3 – parietal;
- 4 – occipital;
- 5 – ventricles, except 4;
- 6 – cerebellum;
- 7 – trunk and 4th ventricle;
- 8 – glioblastoma extending beyond one specified location.
Encryption C71.9 is also sometimes used - unspecified tumor location. When writing an accurate diagnosis, the cytological nature of the neoplasm (glioblastoma) and its clinical manifestation are further indicated: hypertensive-hydrocephalic or vestibular syndrome.
Hypertensive-hydrocephalic syndrome is primarily characterized by excessive accumulation of cerebrospinal fluid in the ventricles and subarachnoid space of the brain. In this regard, the following signs of the disease are observed: attacks of severe headaches, systematic nausea and vomiting, blurred vision, drowsiness, loss of appetite, convulsions and loss of consciousness in severe cases.
Vestibular syndrome manifests itself in a disorder of the motor function of the body. This is expressed in dizziness, confusion and instability of body position (a feeling of movement, rotation of the body, although the person is in a standing or lying position). Like the previous manifestation of cancer, the syndrome may include nausea, vomiting, diarrhea, anxiety, changes in blood pressure and heart rate, since in this case the tumor affects the functioning of the nerve centers of the reticular formation.
As the size of the neoplasm increases, the severity of brain disorders will increase, while at the initial stage there are disturbances in the perception of information by the organs of touch, taste, hearing, and vision; there is a disorder of cognitive functions of the brain. This is expressed in deterioration of memory, perception, difficulty in performing intellectual tasks, mental disorders, even personality degradation.
Against the background of increased intracranial pressure, the patient may experience a hemorrhagic stroke followed by cerebral bleeding, which leads to a sharp deterioration of the condition. In this case, in the absence of medical care, the death of the patient may occur.
At the last stage of development, glioblastoma is manifested by impaired sensitivity, the appearance of convulsions and numbness of the extremities. Paralysis and epilepsy may also develop. This manifestation of the disease is extremely dangerous for the patient’s life, so he simply needs qualified medical care in a hospital setting.
Stages and symptoms
Glioblastoma is a severe pathology that progresses at a high rate. Even timely diagnosis and therapy will not give a 100% positive result and do not guarantee favorable prognosis.
At a young age, secondary glioblastomas are often detected; they grow much more slowly compared to primary ones.
Glioblastoma has its own characteristic features:
- In most cases, it appears in the temporal or frontal lobe of the brain, and can be diagnosed in the brainstem or cerebellum.
- When examined under a microscope, it is clear that glioblastoma cells are atypical, they have no resemblance to healthy ones at all, which is why they grow in size and multiply so quickly.
- The type of tumor growth is diffuse. Glioblastoma rapidly spreads to the brain because it has its own network of blood vessels, and it extremely rarely spreads metastases.
- The consistency of the neoplasm can be soft and hard, and can have different sizes - from a few centimeters to spreading throughout the entire hemisphere.
- Cell infiltration always extends beyond the visible boundaries of glioblastoma.
- An actively growing glioblastoma provokes an increase in intracranial pressure and can lead to hydrocephalus.
Glioblastoma multiforme is characterized by noticeable symptoms even at the very beginning of its development. Signs of pathology largely depend on the location of the tumor.
When it is close to the speech and motor centers, disturbances in the functioning of these systems and frequent fainting appear. With such symptoms, a diagnosis is made quickly.
All manifestations of the development of the disease are classified into 2 groups: focal neurological and cerebral.
General cerebral symptoms of glioblastoma
When glioblastoma occurs, intracranial pressure increases, as the tumor compresses the anatomical structures of the brain. Thus, general cerebral symptoms of glioblastoma include:
- Headache. They progress, are characterized by high intensity, bursting in nature, become stronger in the lying position and in the morning after getting up. The pain cannot be relieved with either conventional or narcotic painkillers; only methods that reduce the level of intracranial pressure will help. The pain may be accompanied by nausea and vomiting, but after this there is no relief.
- Dizziness. They manifest themselves as a result of severe compression of the vestibular nerve and cerebellum. As glioblastoma progresses, the patient cannot even simply turn his head or get out of bed.
- Vomiting of central origin or cerebral origin. It occurs under the influence of irritation of the vomiting center located in the midbrain, with a large tumor size. Every irritant provokes vomiting - these are movements, food and water, medications. For this reason, patients are often transferred to parenteral nutrition.
- Focal neurological symptoms

The degree of manifestation of such signs depends on the location of the oncological focus. There are such groups of focal signs of damage:
- Motor disorders - paralysis of one or several limbs occurs, usually only on one side of the body, which is opposite to the location
- neoplasms. With bilateral paralysis, doctors conclude that the tumor has grown in both hemispheres.
- Sensory dysfunction - there is an increase, decrease or complete disappearance of certain types of sensitivity - temperature, pain, tactile.
- Memory and intelligence deteriorate, a person’s character changes, and multiple mental disorders arise.
- Convulsions with fits.
- Balance and hearing problems; sometimes the patient cannot control the position of his own body.
- The patient ceases to understand oral and written speech and cannot speak on his own.
- Visual impairment.
- Hallucinations.
- Problems in the functioning of the autonomic nervous system - problems with heartbeat, breathing rate.
- The patient may fall into a coma.
The smaller the size of the tumor, the fewer symptoms appear, and the patient has a greater chance of recovery and prolongation of normal life.
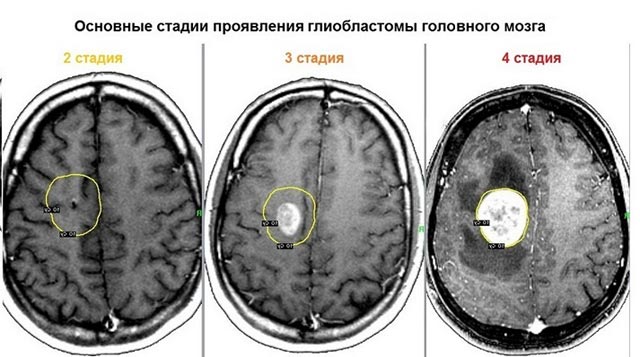
Tumor stages
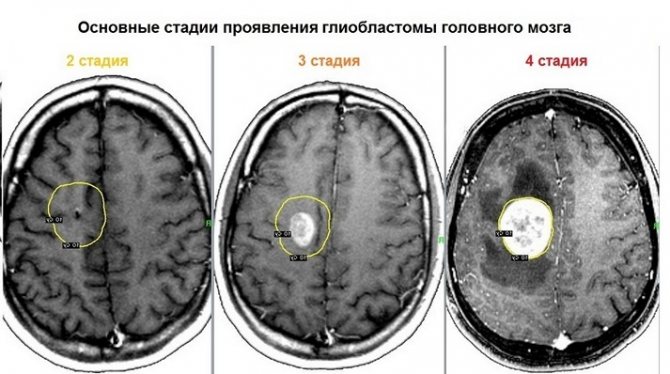
There are four stages of the disease of neoplasm development:
- 1st degree. Almost no manifestations of damage occur. The tumor, in fact, is not yet malignant and is difficult to diagnose.
- 2nd degree. It is characterized by the degeneration of cells into atypical ones. The tumor is still growing very slowly.
- 3rd degree. Necrosis has not yet developed, but atypical cells are rapidly dividing, and the neoplasm is actively progressing.
- 4th degree. Rapid tissue death begins. Stage 4 glioblastoma of the brain has only a negative prognosis.
The exact stage can be established only after a comprehensive diagnosis.
Neuroscience for everyone. Case history: glioblastoma multiforme
Glioblastoma multiforme (GBM) is the most common malignant brain tumor. Its other name is grade IV astrocytoma.
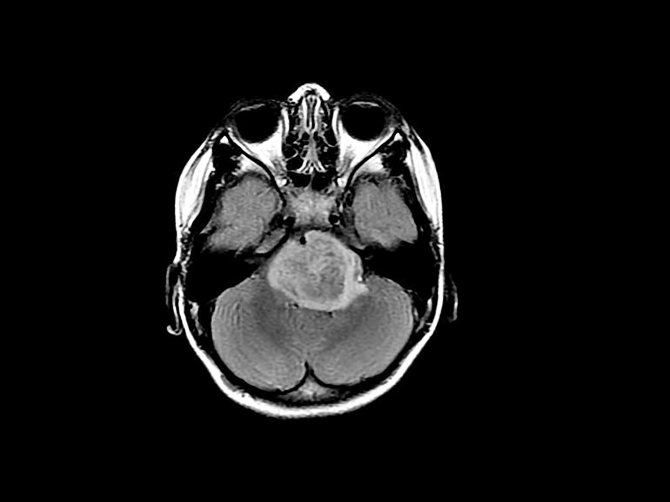
Both names are associated with the type of cells from which the tumor originates: its cause is the malignant degeneration of astrocytes, which belong to glial tissue. Glia are cells that are not involved in the transmission of nerve impulses, but are necessary for neurons to organize the correct structure, protection and other auxiliary functions. Read more about these cells in our separate article. Gliomas are any malignant brain tumors that originate from glial cells. During malignant degeneration, glial cells, in particular astrocytes, lose the characteristics of mature functional cells and become less differentiated. They also lose touch with their neighbors and begin to share uncontrollably.
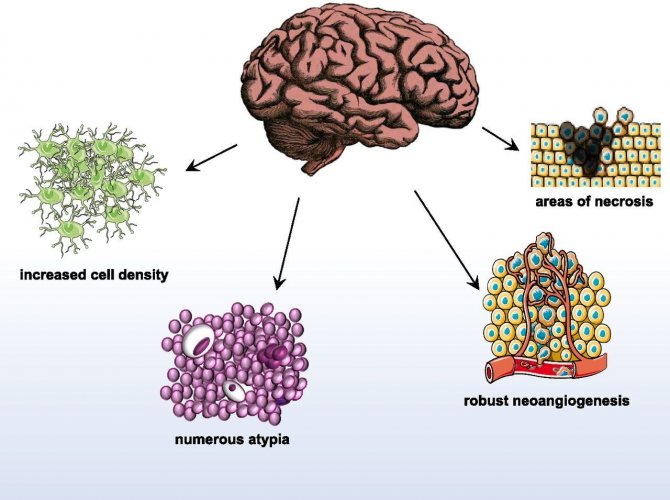
Characteristic features of glioblastoma
Symptoms
The tumor may grow without symptoms for some time, but sooner or later they appear. The most common symptoms are headaches, nausea, seizures, changes in vision, speech, hearing and character. Venous thromboembolism also quite often develops - that is, the formation of clots (thrombi) in the veins.
As a rule, symptoms develop 3-6 months after the appearance of the tumor, but it happens that a less aggressive tumor, which initially grows slowly and asymptomatically, degenerates into glioblastoma. In this case, it may take years for the tumor to manifest itself.
Symptoms depend on which parts of the brain are affected by the tumor and what is the mechanism of its effect on healthy tissue. There are three main mechanisms:
- with necrosis of nervous tissue under the influence of a tumor, neuronal deficiency develops, manifesting itself in different forms depending on the location of the tumor. Thus, in patients with visual and hearing impairments, the tumor is found in the temporal cortex, and with changes in character and thinking disorders - in the frontal regions;
- increased intracranial pressure leads to secondary effects – most often, headaches;
- 20-40% of patients develop seizures, which can be either local or general.
Diagnostics
The tumor is diagnosed using MRI or, then the patient must undergo a biopsy to confirm the diagnosis and clarify the spectrum of mutations.
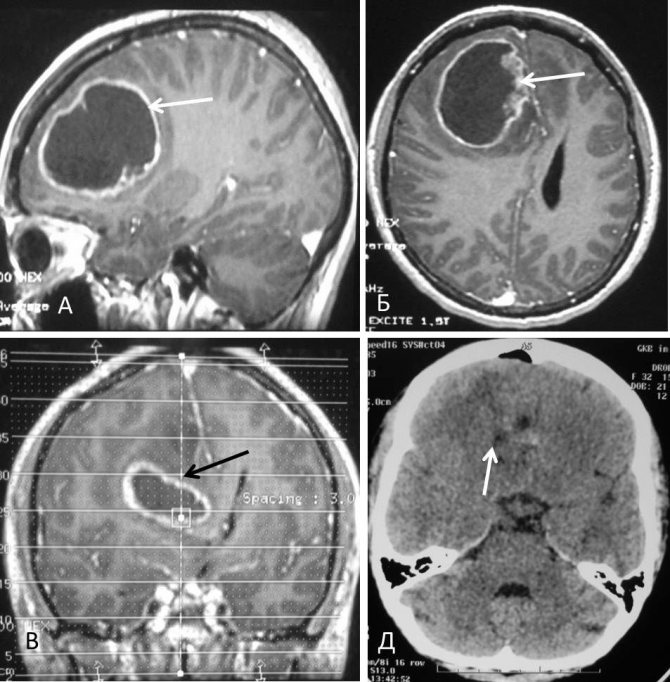
In an MRI image, the tumor appears as a dark spot surrounded by a light border - this is the area of edema.
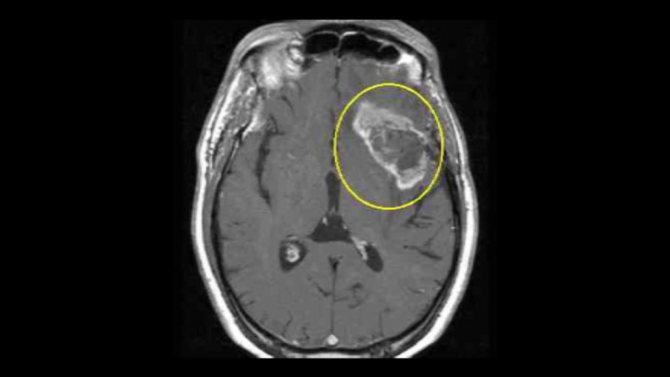
Characteristic appearance of glioblastoma on an MRI image. In the center there is a dark area of necrosis, that is, dead cells, and a light area around the tumor is edema.
Most often, glioblastoma is found in the cerebral hemispheres, but it can also affect other parts of the brain.
Epidemiology
Glioblastoma can occur at any age, but the peak incidence occurs between 55 and 60 years of age.
The incidence of glioblastoma in the USA is approximately 10,000 per year, in the world – more than 28,000. In Russia, approximately 2000-2500 cases of glioblastoma are registered per year.
Forecast
Glioblastoma is the incurable and most aggressive brain tumor, the average life expectancy after surgical removal of the tumor is 15-23 months, and the 5-year survival rate is only 6%. However, in a number of patients it is possible to achieve long-term remission. The more complete the resection, that is, how well the surgeon managed to remove the tumor, the higher the likelihood of a good prognosis. A number of tumor biomarkers and their combinations also influence the prognosis. Their role can be played by genetic markers (for example, mutations in certain genes), and levels of mRNA encoding certain proteins, and levels of the proteins themselves, and some chromosomal rearrangements. There are markers of good and poor prognosis. However, due to the limited number of treatment options (see below), the determination of markers, as well as the results of whole-genome sequencing, is not yet important for the treatment strategy, but is used for research purposes.
Causes
Why glioblastoma occurs is unknown. The risk of its occurrence increases with exposure to radiation and the presence of certain congenital mutations, but neither factor is a reliable predictor. No relationship was found between the risk of developing glioblastoma and factors such as smoking, drug use, eating habits, cell phone use, exposure to household electromagnetic fields, and traumatic brain injury.
The main characteristic feature of glioblastoma, leading to such an aggressive course of the disease and the complexity of therapy, is its heterogeneity. Not only do tumors vary widely in character from patient to patient, but within the same patient, a tumor may be composed of genetically dissimilar cells with very different mutations. In addition, this diversity is constantly changing, especially during the treatment process. Cells exposed to stress accelerate their evolution and develop new ways of survival.
Interestingly, people with allergies have a 40% lower risk of developing gliomas (the name given to all malignant tumors originating from glial cells). This is probably due to the fact that in this case the immune system is in an activated state and does not allow the tumor to grow.
Existing therapy
Despite many efforts to develop new therapies for glioblastoma multiforme, it is still very difficult to treat, and most attempts fail in clinical trials.
The mainstays of treatment for glioblastoma are surgery, radiotherapy and chemotherapy. In addition, symptomatic supportive care, such as corticosteroids to reduce swelling and inflammation and anticonvulsants to reduce seizures, is a very important part of patient care.
The future fate of the patient critically depends on the possibility of performing a surgical operation and the quality of its execution. If the entire tumor can be excised with minimal damage to surrounding tissue, the prognosis can be quite favorable. Unfortunately, in more than 80% of cases this cannot be done because the tumor is too invasive (that is, it invades surrounding healthy tissue).
After surgery, doctors use radiation therapy to destroy any remaining tumor cells. Studies have shown that in elderly patients, surgery followed by radiotherapy significantly prolongs life, increasing the median life expectancy from 2 to 8 months. However, radiotherapy also damages healthy nerve tissue, so its use does not always lead to good results.
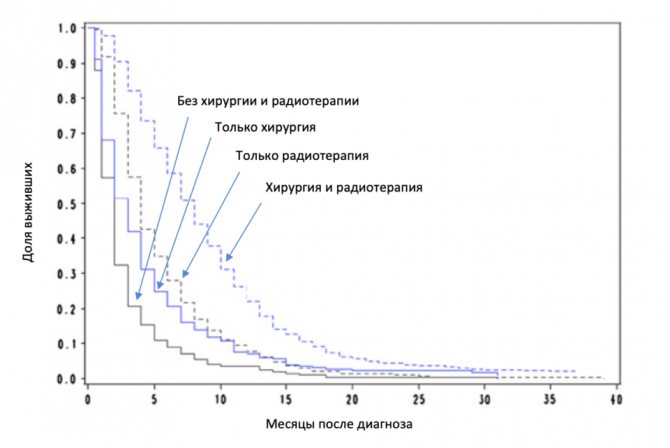
Impact of surgery and radiotherapy on survival in elderly patients.
The standard chemotherapy for newly diagnosed patients is temozolomide. It methylates guanine in DNA. As a result, the cellular DNA repair mechanism is unable to repair DNA strand breaks at these locations. Free DNA ends appear, and since their presence is irremovable, this is a signal for the cell to undergo apoptosis - controlled cell death.
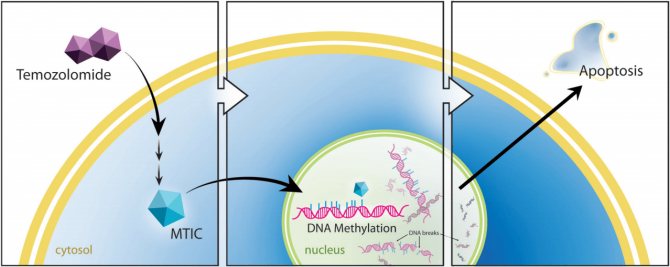
Mechanism of action of temozolomide. Once in the cell, temozolomide is converted into the alkylating agent MTIC (5-(3-methyltriazen-1-yl)-imidazol-4-carboximide), which methylates guanine DNA, which leads to cell apoptosis.
True, there are several mechanisms that protect tumor cells from the action of temozolomide and other similar agents. One of the main mechanisms in glioblastoma is increased levels of the enzyme methylguanine methyltransferase (MGMT), which eliminates guanine methylation. In addition, since healthy cells also suffer from the effects of methylation, although to a lesser extent than tumor cells, the use of temozolomide is limited due to undesirable effects, i.e. toxicity.
However, surgery followed by radiotherapy and temozolomide has become the standard treatment for newly diagnosed glioblastoma due to its proven effect on increasing life expectancy.
Other chemotherapy agents that are typically used in patients with recurrent glioblastoma (so-called recurrent glioblastoma): carmustine, lomustine, carboplatin, oxaliplatin, irinotecan, etoposide - are also quite toxic.
Many chemotherapy agents cannot cross the blood-brain barrier and therefore do not reach the brain in sufficient concentrations to have a therapeutic effect.
In the United States, the FDA approved Roche's Avastin bevacizumab for the treatment of patients with recurrent glioblastoma in 2009). This is an antibody that binds to the blood plasma protein VEGF (vascular endothelial growth factor, vascular endothelial growth factor). Since tumors constantly require nutrients, tumor cells produce VEGF, which leads to the growth of blood vessels into the tumor.
Avastin has been successfully used to treat a number of cancers. True, in glioblastoma it did not show the most outstanding results: although Avastin slightly increases the time to progression, it does not affect the survival of patients. Because of this, it has not been registered in Europe, although it may be used off-label (that is, not for a registered indication) there. A study of Avastin in patients with newly diagnosed glioblastoma in combination with radiotherapy and temozolomide also did not show a beneficial effect on survival.
It is obvious that more and more new means of therapy are required for both newly diagnosed and recurrent glioblastoma. For recurrent glioblastoma, the prognosis is especially dismal, with a median survival of only 6.2 months. For some types of cancer, scientists in recent decades have developed many approaches that have made it possible to significantly extend the life expectancy of patients, and in some cases, cure them. However, in the field of glioblastoma, such success has not yet been achieved, as already mentioned. Due to their heterogeneity and location in the brain, these tumors are very difficult to treat, and failure of new therapies is unfortunately common. Let's talk about some of them.
Failed therapy
Anti-EGFR agents
EGFR (epidermal growth factor receptor, epidermal growth factor receptor) is a cell surface protein that, upon binding of a ligand (extracellular signaling protein), triggers a cascade of intracellular processes leading to cell division, growth, migration and increased survival. Agents directed against EGFR either interfere with the binding of the ligand to the receptor (antibodies cetuximab, panitumamab and others) or inhibit (that is, suppress) the activity of EGFR inside the cell (erlotinib, gefitinib, osimertinib). These drugs are successfully used to treat cancers of the lung, head and neck (these types of cancer do not include brain tumors) and some others.
In glioblastoma, the most common variant, in 30% of patients, is the EGFR v III variant, in which the extracellular part is shortened. Such a protein acquires the ability to turn on intracellular processes regardless of ligand binding, which increases the aggressiveness of tumor cells.
Studies of available EGFR inhibitors in patients with glioblastoma have been unsuccessful. The most likely reason is the blood-brain barrier (BBB), which prevents substances from penetrating the tumor. The presence of a tumor somewhat disrupts the BBB, but this is not enough to provide a therapeutic effect. There are now new anti-EGFR agents in early-stage clinical trials that cross the BBB. Whether they will be sufficiently effective remains to be seen.
Also in development is a conjugate of an anti-EGFR antibody with a toxin, Depatuxizumab mafodotin. The idea is that the antibody will bind to EGFR on the surface of the tumor cell, then the antibody-EGFR complex will be drawn into the cell, and there the released toxin will kill the cell.
This antibody conjugate against another receptor, HER2, is already used to treat breast cancer, but there have been many failures of similar drugs, mainly due to toxicity. The drug is still in phase 3 in patients with newly diagnosed glioblastoma. In patients with recurrent glioblastoma, it showed a trend toward improved survival (median survival was 9.6 months vs. 8.2 in controls), but it did not reach statistical significance (and therefore is highly likely to be due to chance), so the company decided for now Do not use the drug in these patients.
Rindopepimut
For a long time, hopes were placed on Rindopepimut, a vaccine against EGFR vIII. It is a peptide of 14 amino acids corresponding to the mutated region of EGFR vIII, with keyhole limpet hemocyanin (KLH) attached to it (an adjuvant, that is, a substance that enhances the immune response to the administered antigen). Rindopepimut has been shown to be effective in animals, able to induce an immune response against EGFR vIII, and safe in humans. In two multicenter phase 2 clinical trials in combination with temozolomide in patients with newly diagnosed glioblastoma, Rindopepimut showed very good results with a median patient survival of more than 2 years. The study was a single-arm trial, but because similar patients had previously had a median survival of 16 months, the company moved confidently into Phase 3. However, the vaccine showed no benefit over the control group in the double-blind, randomized trial.
Apparently, one of the reasons for failure is the rapid loss of EGFR protein by tumor cells during therapy: researchers observed such loss in 67-82% of patients. This is logical, since those cells that can do without the EGFR protein and do not express it on the surface are not subject to the action of the immune system and receive a selective advantage. The vaccine stops working and the tumor continues to grow using cells without EGFR.
However, why then were such good results obtained in non-comparative studies? It was already mentioned above that the prognosis is strongly influenced by tumor biomarkers, that is, how elevated the levels of certain proteins are in it and whether there are mutations in them. The Rindopepimut studies did not examine these markers, and it is likely that those studies included patients with a good prognosis. Thus, it has been shown that although the five-year survival rate of patients averages 6%, in patients with a certain combination of markers it reaches 40%! A useful lesson to draw from this is that the results of studies in the field of glioblastoma cannot be trusted unless there is a control group and the most important markers that influence survival are not identified.
It also becomes clear that approaches aimed at one or even several targets will most likely not work in glioblastoma, since tumor cells always find a way to get rid of the target and do without it. And those proteins that no cell can do without are not suitable as targets, since substances that act on them will also affect healthy cells, which will lead to unacceptable toxicity.
Checkpoint inhibitors (checkpoint inhibitors)
This class of therapy targets receptors on the surface of immune system cells or corresponding ligands on the surface of tumor cells. Receptors that are responsible for reducing the activity of the immune system are called checkpoints (from the English checkpoint - control point). Accordingly, blocking these receptors with antibodies takes the brakes off the immune system, and it can attack the tumor, since some tumor proteins are foreign to the body. In this sense, glioblastoma is a good target, since it contains a lot of mutated and unusual proteins.
One of the checkpoint inhibitors, the antibody against the PD-1 receptor on the surface of T-lymphocytes, nivolumab, and other similar agents have already revolutionized the treatment of melanoma, lung cancer and some other types of cancer, as they lead to a cure of 20-30% of patients on treatment. late stages of diseases. However, the use of nivolumab in recurrent glioblastoma has not been successful. The likely cause was the presence of the blood-brain barrier and the multiple mechanisms that the tumor develops to suppress the immune response against itself, in addition to PD-L1/PD-L2.
Studies in patients with newly diagnosed glioblastoma are still ongoing, and there is hope that combinations of nivolumab with other agents will work.
Many other therapies have failed in phase 3 despite showing very good results in phase 2, forcing researchers to be very careful about the study design of all new products. In particular, in the studies of the first two phases, patients undergo a rather strict selection - patients with a poor prognosis, those who had a poor resection, and those who are too elderly are not included. When moving to phase 3, the criteria are relaxed, and the new therapy no longer has an effect on the wider population.
Approaches based on oncolytic viruses—special viral constructs that should infect predominantly tumor cells and destroy them—have not yet shown effectiveness in treating glioblastoma. Approaches based on modified lymphocyte T cells (CAR-T) are still at an early stage of development, and it is too early to talk about their effectiveness.
Several more substances have moved from phase 1-2 to phase 3 clinical trials despite the fact that early studies were non-comparative, and the drugs showed results there that were not much better than the literature data. This is, for example, the alkylating agent VAL-083, the proteasome inhibitor Marizomib.
Promising therapy
Let's now talk about approaches that may still prove successful in the future.
TTF
One of the promising directions in the treatment of glioblastoma is the use of alternating electromagnetic fields. A device that uses this principle is called TTF (Tumor treating fields). It turned out that a field with a frequency of 200 kHz has the strongest effect on the protein tubulin, which makes up microtubules inside cells. Therefore, the field disrupts the process of cell division, which leads to its death (Fig. 9. Here and elsewhere in the figure, a more detailed explanation of the mechanism is provided). Rapidly dividing cells are most susceptible to this, and since the device affects the tumor area, healthy cells are almost not affected.
In a pilot clinical trial, TTF increased survival by 2-fold compared with historical controls in both patients with newly diagnosed and recurrent glioblastoma.
In a comparative phase 3 study in patients with recurrent glioblastoma, TTF again failed to outperform chemotherapy in terms of median survival and time to progression, but because it had virtually no adverse effects, it was approved by the FDA in 2011. A post-marketing study also showed that TTF is at least as good as existing approaches.
In patients with newly diagnosed glioblastoma, the TTF approach statistically significantly prolonged survival in a phase 3 study from 15.6-16.6 months in the control group (standard of care temozolomide) to 19.6-20.5 months in the TTF group. Based on this study, the FDA approved the device for these patients as well.
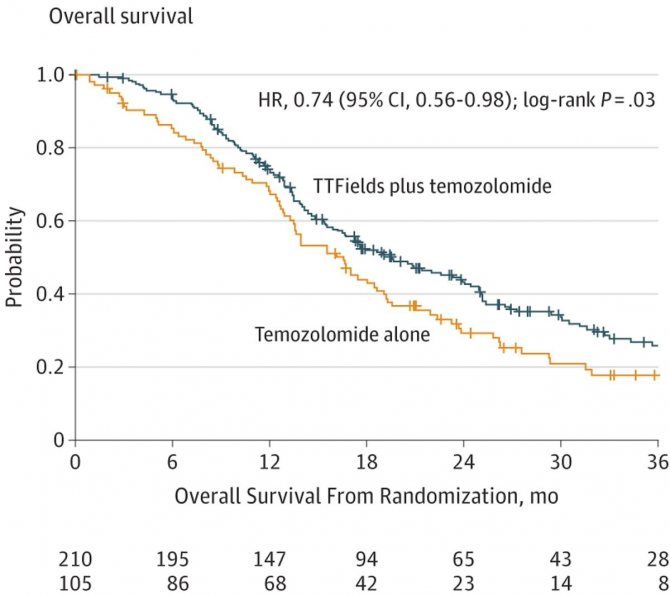
Overall survival graph of patients with newly diagnosed glioblastoma in the TTF + temozolomide (blue curve) and temozolomide (yellow curve) group.
Research on TTF is ongoing, including in combination with various other chemotherapy and targeted drugs, and hopefully, due to its high safety and good effectiveness, this method will take a place in the standard therapy for glioblastoma.
DCVax-L
The dendritic cell vaccine DCVax-L is produced from a patient's tumor cells taken through a biopsy. The cells are then lysed (destroyed) and mixed with dendritic cells obtained from human blood.
Dendritic cells are an important part of the immune system; they are responsible for training the acquired immune system to attack foreign agents. Dendritic cells absorb foreign proteins, then migrate to the lymph nodes and there exhibit fragments of these proteins on their surface. There is a selection of T cells that recognize the presented antigens well. Trained T cells can migrate into the tumor and attack cells carrying foreign proteins.
Thus, tumor cells taken from a patient provide an opportunity for dendritic cells to present that patient's entire repertoire of tumor antigens to T cells, which is expected to make it more difficult for the tumor to develop resistance.
In Phase 1/2, the vaccine showed impressive results: median survival reached a full three years, and two patients lived more than 10 years! In 2006, the company launched a randomized, placebo-controlled, double-blind Phase 3 study.
The study is still progressing despite ongoing challenges for both the study and Northwest Biotherapeutics. The study was stopped several times, first by the FDA, then by the company. Compared to the original plan, the study was redone from phase 2 to phase 3, instead of 141 patients they decided to recruit 341, and the primary criterion of the study was changed. All these changes have not been sufficiently justified by the company and there are concerns that they indicate a high level of uncertainty for the company's success.
On the other hand, the company constantly releases press releases and publications that create the appearance of success: for example, in June 2020, an article was published from which it may seem that the vaccine significantly prolongs the lives of patients. In fact, if you carefully study the article, it shows that the median survival for all groups of patients was 23.1 months, which is not too different from the value known from the literature. But most importantly, since the study is not over yet, neither the doctors nor the company should yet know what the survival rate is in the experimental and control groups.
The company was previously accused of hiding data and misleading investors, and the head of the company is also accused of ordering research at an inflated price from a company that belongs to it.
Even if the vaccine turns out to be successful and is registered, it will not be suitable for all patients: it requires 1-2 grams of tumor to be produced, and less than half of the patients from whom it is possible to extract that much material.
Gene therapy
Tocagen's product is a combination of two drugs: a precursor to the chemotherapy drug 5-fluorouracil (Toca-FC) and a retroviral vector encoding the enzyme cytosine deaminase (Toca 511). The idea is that Toca 511 is first injected, it infects the tumor cells, and the enzyme begins to be produced there. The patient is then given Toca-FC, which is distributed throughout the body, but is converted into an active substance only in tumor cells and selectively kills them.
In phases 1 and 2, the drug showed good results in patients with recurrent glioblastoma, with a median survival of 13.6 months. However, these results were compared not with direct control, but with historical control, where the median was 8.3 months. A phase 3 study is currently underway, with results expected by the end of 2020.
Conclusion
Progress in the treatment of glioblastoma, as in other therapeutic areas, requires enormous efforts to better understand the mechanisms of pathology, discover new targets and diagnostic methods that allow the disease to be detected as early as possible. As mentioned, in the field of glioblastoma the situation is complicated by the fact that it is one of the most heterogeneous tumors: its manifestations differ not only between people, but also in one person different cells can belong to different types, and even evolve over time.
Those approaches that are now in late stages of development will not be able to completely solve the problem of glioblastoma, but perhaps they will prolong the life of some patients, or even cure some of them. It is difficult to say which technology (or combination of technologies) will be the one that will revolutionize the treatment of glioblastoma. Maybe some kind of immunotherapy, such as a vaccine or an oncolytic virus. Maybe some kind of gene therapy or, for example, nanoparticles that can deliver the necessary antibodies beyond the blood-brain barrier. Or maybe some new technology, unlike anything that exists now.
One thing is clear: the path to getting rid of this terrible disease lies in fundamental research by hundreds of scientific teams around the world, in the painstaking work of academic and industrial laboratories, where the mosaic of future knowledge is assembled piece by piece.
Text: Ilya Yasny
Source link
Treatment methods for glioblastoma
Treatment of glioblastoma of the brain involves several methods:
- Chemotherapy. This is a fairly effective method to treat this type of oncology. Medicines and their dosage are selected in accordance with the degree of damage, the general condition of the patient, his age and other additional factors. Chemotherapy causes the death of cancer cells. Modern medications have a minimal effect on the bone marrow and healthy cells, and therefore do not cause complications.
- Radiation therapy and radiosurgery. Only an integrated approach can cure glioblastoma, so radiation is used simultaneously with chemotherapy. It should be started after surgery to remove the tumor. Radiation completely replaces surgery when the tumor is inoperable. The course of radiation therapy is 6-8 sessions over 5 days or longer. But it is possible to achieve tumor regression after irradiation only in 20% of cases.
- Maintenance therapy. Special antitumor drugs are often prescribed as maintenance treatment. They must be taken for one month after completion of radiation therapy. Take the tablets for 6 short courses of treatment over 5 days with breaks of 20-25 days. Therapy is aimed at prolonging the patient's life.
- Operation. In most cases, glioblastoma is considered inoperable, so it is not removed, but treated with conservative methods. When removal surgery is permissible, special rehabilitation is required after its completion. Surgery can prolong a person's life.
- Folk remedies. Despite the high rate of progression of glioblastoma and the threat to life, some hope for the help of alternative treatment. In fact, such methods of control are more suitable for preventing tumor relapses. For example, radish juice is rubbed into the scalp for 20-30 minutes. Then you need to leave the compress overnight. Procedures are carried out weekly.
Even with timely organization of effective treatment, it is unlikely that a complete recovery will be achieved. The method in which the prognosis can be as positive as possible is surgical removal.

There are also certain nutritional rules for glioblastoma of the brain. The diet should contain many foods with calcium and magnesium, as these microelements contribute to the rapid recovery of the body after chemotherapy.
It is strictly forbidden to consume the following products:
- sauerkraut;
- mustard;
- dried fruits;
- water with gases;
- legumes;
- dairy products.
Diagnosis of glioblastoma of the brain.
A mandatory test is an MRI with contrast.
According to magnetic resonance imaging, only the presence of glial formation can be revealed. An accurate diagnosis can only be made after surgery or biopsy followed by histological examination of tumor particles. Histological diagnosis is necessary to determine the treatment strategy in the postoperative period in the amount of radiation and chemotherapy.
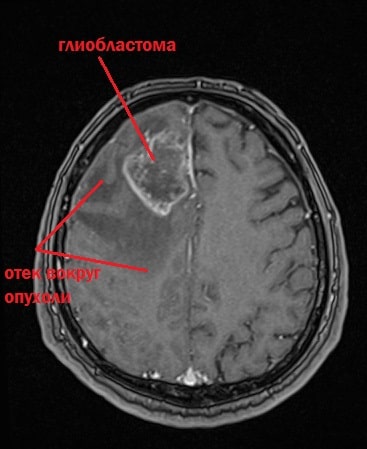
Glioblastoma mri photo.
Auxiliary diagnostic methods:
- CT with intravenous contrast is performed in the presence of contraindications to MRI and as a control after surgery in the first 24-72 hours;
- EEG is used for epilepsy;
- Positron emission tomography (PET) with methionine is performed for recurrent glioblastoma after radiation therapy for differential diagnosis with radiation necrosis;
- Immunohistochemical examination of the tumor is carried out in case of doubt about the results of histological examination;
- Fundus examination by an ophthalmologist to assess intracranial hypertension.